| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
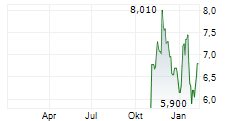
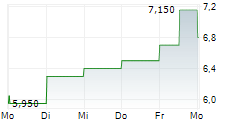
| 7,150 | 7,400 | 27.02. |
Chart-Typ:
Zeitraum:
| Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen |
| Structure Therapeutics Inc.: Structure Therapeutics Announces Initiation of Phase 1 Clinical Study of Oral Small Molecule Amylin Receptor Agonist ACCG-2671 for the Treatment of Obesity | SAN FRANCISCO, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Structure Therapeutics Inc. (NASDAQ: GPCR), a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for... ► Artikel lesen |
| Structure Therapeutics Inc.: Structure Therapeutics Announces Closing of Upsized $747.5 Million Public Offering of ADSs and Pre-Funded Warrants and Full Exercise of the Underwriters' Option to Purchase Additional ADSs | SAN FRANCISCO, Dec. 11, 2025 (GLOBE NEWSWIRE) -- Structure Therapeutics Inc. (NASDAQ: GPCR), a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for... ► Artikel lesen |
| Weekly Buzz: MGNX's LINNET Trial On Hold; Eton Pharmaceuticals, ALUR Get FDA Nod; GILD Snaps Up ACLX | FOSTER CITY (dpa-AFX) - This week, the biotech space witnessed significant milestones, including FDA approvals, oncology drug acquisitions, and collaborations. Positive clinical trial results... ► Artikel lesen |
| ROUNDUP/Aktien New York Schluss: Klare Verluste - KI-Ängste und Zölle belasten | NEW YORK (dpa-AFX) - Die US-Aktienmärkte haben einen Fehlstart in die neue Börsenwoche hingelegt. Erneute Sorgen um die Auswirkungen Künstlicher Intelligenz (KI) auf die Unternehmensgewinne sowie die... ► Artikel lesen |
| Aktien New York: Schwacher Wochenstart - KI-Ängste und Zölle belasten | NEW YORK (dpa-AFX) - Die US-Aktienmärkte sind mit klaren Verlusten in die neue Börsenwoche gestartet. Erneute Sorgen um die Auswirkungen Künstlicher Intelligenz (KI) auf die Unternehmensgewinne sowie... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SHANGHAI HENLIUS BIOTECH INC finden Sie auf Wallstreet Online.