| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
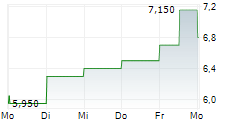
| 7,150 | 7,400 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - PERTUZUMAB BIOSIMILAR HLX11 (RECOMBINANT ANTI-HER2 DOMAIN II HUMANIZED MONOCLONAL ANTIBODY INJECTION) RECEIVED ... | - | HKEx | ||
| Fr | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - FIRST PATIENT HAS BEEN DOSED IN THE PHASE 2/3 CLINICAL STUDY OF HLX22 (RECOMBINANT HUMANISED ANTI-HER2 MONOCLONAL ... | 1 | HKEx | ||
| Di | HENLIUS (02696): INSIDE INFORMATION ANNOUNCEMENT - AMENDMENTS TO LICENSE AGREEMENTS WITH ABBOTT AND KGBIO FOR HANSIZHUANG | 1 | HKEx | ||
| SHANGHAI HENLIUS BIOTECH Aktie jetzt für 0€ handeln | |||||
| 12.02. | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - THE INVESTIGATIONAL NEW DRUG APPLICATION OF HLX15-SC (RECOMBINANT ANTI-CD38 FULLY HUMAN MONOCLONAL ANTIBODY ... | - | HKEx | ||
| 11.02. | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - FIRST PATIENT DOSED IN A PHASE 1B/2 CLINICAL STUDY OF HLX43 FOR INJECTION (AN ANTI-PD-L1 ANTIBODY-DRUG CONJUGATE) ... | - | HKEx | ||
| 06.02. | Eisai, Henlius Strike Japan Commercialization Deal for PD-1 Antibody Serplulimab | 3 | Contract Pharma | ||
| 06.02. | Eisai, Henlius partner on anti-PD-1 antibody in Japan | 2 | Investing.com | ||
| 06.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 422 | JCN Newswire | TOKYO and SHANGHAI, Feb 6, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Shanghai Henlius Biotech, Inc. (Headquarters: Shanghai, China, CEO: Jason Zhu... ► Artikel lesen | |
| 05.02. | Eisai signs $388m deal for Japanese rights to Henlius' anti-PD-1 mAb | 3 | Pharmaceutical Technology | ||
| 05.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 420 | PR Newswire | TOKYO and SHANGHAI, Feb. 5, 2026 /PRNewswire/ -- Eisai Co., Ltd. ("Eisai") and Shanghai Henlius Biotech, Inc. ("Henlius") today announced the conclusion of an exclusive commercialization... ► Artikel lesen | |
| 05.02. | HENLIUS (02696): INSIDE INFORMATION ANNOUNCEMENT - LICENSE AGREEMENT WITH EISAI IN RESPECT OF HANSIZHUANG | 1 | HKEx | ||
| 04.02. | HENLIUS (02696): NEXT DAY DISCLOSURE RETURNS | 1 | HKEx | ||
| 04.02. | HENLIUS (02696): INSIDE INFORMATION ANNOUNCEMENT - COMPLETION OF THE H SHARE FULL CIRCULATION | - | HKEx | ||
| 27.01. | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - APPLICATION FOR CLINICAL TRIAL OF HLX43 FOR INJECTION (AN ANTI-PD-L1 ANTIBODY-DRUG CONJUGATE) IN COMBINATION ... | - | HKEx | ||
| 20.01. | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - INVESTIGATIONAL NEW DRUG APPLICATION FOR A PHASE 1B/2 CLINICAL TRIAL OF HLX701 (RECOMBINANT HUMAN SIRPA - IGG4 ... | - | HKEx | ||
| 19.01. | HENLIUS (02696): INSIDE INFORMATION ANNOUNCEMENT - LISTING APPROVAL GRANTED BY THE STOCK EXCHANGE FOR THE H SHARE FULL CIRCULATION OF THE COMPANY | 1 | HKEx | ||
| 16.01. | Henlius Showcases "Globalisation 2.0" Strategy and Mid-to-Long-Term Innovation Blueprint at JPM 2026 | 254 | PR Newswire | SAN FRANCISCO, Jan. 16, 2026 /PRNewswire/ -- The 44th J.P.Morgan Healthcare Conference (JPMHC) was successfully held in San Francisco, the United States, from January 12 to 15. On January... ► Artikel lesen | |
| 16.01. | HENLIUS (02696): INSIDE INFORMATION ANNOUNCEMENT - ISSUANCE OF THE FILING NOTICE BY THE CSRC FOR THE H SHARE FULL CIRCULATION BY THE COMPANY | 2 | HKEx | ||
| 13.01. | HENLIUS (02696): VOLUNTARY ANNOUNCEMENT - BIOLOGICS LICENSE APPLICATION (BLA) FOR HANBEITAI (BEVACIZUMAB INJECTION) HAS BEEN ACCEPTED BY THE UNITED STATES ... | 1 | HKEx | ||
| 31.12.25 | HENLIUS (02696): POLL RESULTS OF THE RESOLUTION PROPOSED AT THE 2025 FOURTH EXTRAORDINARY GENERAL MEETING HELD ON WEDNESDAY, 31 DECEMBER 2025 | 3 | HKEx |
1 2 3 4 Weiter >>
66 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| RELAY THERAPEUTICS | 10,250 | +12,02 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | -3,48 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| KYMERA THERAPEUTICS | 91,36 | -3,85 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| COGENT BIOSCIENCES | 38,870 | -1,55 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | ||
| EDGEWISE THERAPEUTICS | 30,450 | +2,77 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen |
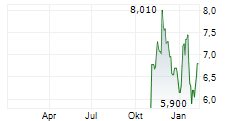
SHANGHAI HENLIUS BIOTECH INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,050 (-0,69 %) liegt der Kurs bei 7,150 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SHANGHAI HENLIUS BIOTECH INC finden Sie auf Wallstreet Online.