- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Forecasting The Future: 4 Analyst Projections For Arcutis Biotherapeutics | 3 | Benzinga.com | ||
| Do | Needham raises Arcutis stock price target to $36 on strong sales | 2 | Investing.com | ||
| Do | H.C. Wainwright raises Arcutis stock price target on strong Zoryve sales | 2 | Investing.com | ||
| Do | Starke Zoryve-Verkäufe: H.C. Wainwright hebt Kursziel für Arcutis an | 2 | Investing.com Deutsch | ||
| Do | Arcutis Q4 2025 slides: ZORYVE drives profitability, revenue beats | 3 | Investing.com | ||
| ARCUTIS BIOTHERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| Mi | Arcutis Biotherapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 1 | SEC Filings | ||
| Mi | Arcutis Biotherapeutics, Inc. - 10-K, Annual Report | 2 | SEC Filings | ||
| Mi | Arcutis Biotherapeutics, Inc.: Arcutis Announces Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | 128 | GlobeNewswire (Europe) | Q4 2025 net product revenue for ZORYVE (roflumilast) was $127.5 million, an 84% increase compared to Q4 2024, and a 29% increase compared to Q3 2025Full year 2025 net product revenue for ZORYVE was... ► Artikel lesen | |
| Mi | Arcutis Biotherapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| Di | Preview: Arcutis Biotherapeutics' Earnings | 1 | Benzinga.com | ||
| Di | Arcutis Biotherapeutics, Inc.: Professional Golfer Max Homa Joins Arcutis' Free to Be Me Campaign, Urging Individuals with Seborrheic Dermatitis to Tee Up a Conversation with Their Healthcare Provider About Long-Term Treatments | 2 | GlobeNewswire (USA) | ||
| 17.02. | Arcutis Biotherapeutics to Report Q4 Earnings: What's in the Cards? | 4 | Zacks | ||
| 11.02. | Arcutis auf dem Guggenheim Summit: Strategisches Wachstum und Marktexpansion | 7 | Investing.com Deutsch | ||
| 05.02. | Arcutis Biotherapeutics, Inc.: Arcutis Biotherapeutics Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 3 | GlobeNewswire (USA) | ||
| 02.02. | Arcutis reports positive results for eczema cream in infants | 4 | Investing.com | ||
| 02.02. | Arcutis Biotherapeutics, Inc.: Arcutis Announces Positive Topline Results for INTEGUMENT-INFANT Phase 2 Trial of ZORYVE (roflumilast) Cream 0.05% in Infants with Mild to Moderate Atopic Dermatitis | 144 | GlobeNewswire (Europe) | 58% of participants achieved a 75% improvement in Eczema Area and Severity Index (EASI-75) with ZORYVE cream 0.05% at Week 4Investigational ZORYVE cream 0.05% was well tolerated and demonstrated a... ► Artikel lesen | |
| 26.01. | Arcutis, Kowa part ways on US marketing partnership for Zoryve | 6 | FiercePharma | ||
| 26.01. | Arcutis terminates promotion agreement with Kowa for ZORYVE | 2 | Investing.com | ||
| 26.01. | Arcutis Biotherapeutics, Inc. Announces Termination of Promotion Agreement with Kowa | 4 | GlobeNewswire (USA) | ||
| 21.01. | Arcutis Biotherapeutics, Inc.: Nationwide Survey Underscores Concerns with Use of Topical Steroids and Need for Long-Term Treatment Strategies Facing the 46 Million Americans with Chronic Inflammatory Skin Conditions | 3 | GlobeNewswire (USA) |
1 2 3 4 Weiter >>
68 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| CRISPR THERAPEUTICS | 50,50 | -0,98 % | Crispr Therapeutics gains amid takeover speculation |
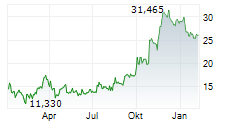
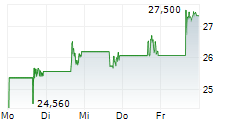
ARCUTIS BIOTHERAPEUTICS INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -1,585 (-5,55 %) liegt der Kurs bei 26,985 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ARCUTIS BIOTHERAPEUTICS INC finden Sie auf Wallstreet Online.