| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 659,60 | 661,00 | 03.03. | |
| 0,000 | 0,000 | 03.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Sanofi (SNY) and Regeneron's Dupixent Recommended for EU Approval | 5 | Insider Monkey | ||
| REGENERON PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| Mo | If You Invested $1000 In Regeneron Pharmaceuticals Stock 20 Years Ago, You Would Have This Much Today | 2 | Benzinga.com | ||
| Mo | RBC Capital erhöht Kursziel für Regeneron aufgrund von Melanom-Daten-Ausblick | 6 | Investing.com Deutsch | ||
| Mo | RBC Capital raises Regeneron stock price target to $765 on melanoma data outlook | 3 | Investing.com | ||
| Sa | Is Regeneron Pharmaceuticals Stock Underperforming the Nasdaq? | 7 | Barchart.com | ||
| Fr | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | 458 | Dow Jones News | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| Fr | Regeneron, Sanofi Receive Positive CHMP Opinion For Dupixent In Pediatric CSU | 326 | AFX News | PARIS (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) and Sanofi SA (SNY) Friday said the European Medicines Agencys Committee for Medicinal Products for Human Use (CHMP) has issued a positive... ► Artikel lesen | |
| Fr | Sanofi: Sanofi and Regeneron's Dupixent recommended for EU approval to treat chronic spontaneous urticaria in young children with ongoing symptoms despite treatment | 618 | GlobeNewswire (Europe) | Sanofi and Regeneron's Dupixent recommended for EU approval to treat chronic spontaneous urticaria in young children with ongoing symptoms despite treatment
If approved, Dupixent would be the first... ► Artikel lesen | |
| Fr | Regeneron Pharmaceuticals, Inc.: Dupixent (dupilumab) Recommended for EU Approval to Treat Chronic Spontaneous Urticaria (CSU) in Young Children with Ongoing Symptoms Despite Treatment | 521 | GlobeNewswire (Europe) | If approved, Dupixent would be the first targeted medicine in the EU indicated for children aged 2 to 11 years with CSU inadequately controlled by standard-of-care antihistamine treatment CSU is... ► Artikel lesen | |
| Do | Regeneron Pharmaceuticals, Inc.: Regeneron Renews Sponsorship of the Regeneron Science Talent Search Through 2036, Committing an Additional $150 Million to Empower the Next Generation of Science and Technology Leaders | 5 | GlobeNewswire (USA) | ||
| 25.02. | FDA Approves Sanofi And Regeneron's Dupixent As First Medicine For Allergic Fungal Rhinosinusitis | 461 | AFX News | PARIS (dpa-AFX) - Sanofi (SNY, SAN.PA) said FDA has approved Dupixent or dupilumab for the treatment of adult and pediatric patients aged 6 years and older with allergic fungal rhinosinusitis... ► Artikel lesen | |
| 25.02. | 2 Reasons Regeneron Stock Could Crush the Market for the Next 10 Years | 5 | The Motley Fool | ||
| 24.02. | Regeneron, Sanofi Blockbuster Dupixent Scores FDA Nod For Rare Sinus Condition | 13 | Benzinga.com | ||
| 24.02. | Regeneron, Sanofi win Dupixent label expansion for allergic fungal rhinosinusitis | 5 | Seeking Alpha | ||
| 24.02. | Regeneron Pharmaceuticals, Inc.: Dupixent (dupilumab) Approved in the U.S. as the First and Only Medicine for Allergic Fungal Rhinosinusitis (AFRS) | 279 | GlobeNewswire (Europe) | Approval in adults and children aged 6 years and older supported by Phase 3 trial demonstrating Dupixent significantly reduced nasal signs and symptoms, and systemic corticosteroid use or surgery... ► Artikel lesen | |
| 24.02. | Sanofi: Sanofi and Regeneron's Dupixent approved in the US as the first and only medicine for allergic fungal rhinosinusitis | 1.923 | GlobeNewswire (Europe) | Sanofi and Regeneron's Dupixent approved in the US as the first and only medicine for allergic fungal rhinosinusitis
Approval in adults and children aged 6 years and older supported by phase 3 study... ► Artikel lesen | |
| 24.02. | FDA Accepts Regeneron (REGN) BLA for Garetosmab Under Priority Review | 3 | Insider Monkey | ||
| 20.02. | Dividendenbekanntmachungen (20.02.2026) | 9.535 | wallstreetONLINE (Dividenden) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) ASX LIMITED AU000000ASX7 1,018 AUD 0,6103 EUR ATMUS FILTRATION TECHNOLOGIES INC US04956D1072 0,055 USD 0,0467 EUR AUDIOCODES LTD IL0010829658 0... ► Artikel lesen | |
| 20.02. | FDA starts review of Regeneron's drug for rare disease FOP | 7 | pharmaphorum | ||
| 19.02. | Regeneron Pharma Says FDA Accepts Garetosmab BLA For Priority Review For Adults With FOP | 388 | AFX News | WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) announced Thursday that the U.S. Food and Drug Administration (FDA) has accepted for Priority Review the Biologics License Application... ► Artikel lesen |
1 2 3 4 5 Weiter >>
331 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 86,55 | -0,23 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,304 | -6,72 % | Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen | |
| BB BIOTECH | 50,20 | -3,83 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| MEDIGENE | 0,035 | -10,20 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 40,700 | -1,44 % | Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 42,915 | +0,05 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,060 | -35,21 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,448 | +0,41 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 324,80 | +0,06 % | Amgen EVP Sold $20.77M In Company Stock | ||
| EPIGENOMICS | 0,960 | -5,88 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,122 | -0,62 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,70 | -0,12 % | Stryker highlights new additions to Mako, Triathlon portfolios at AAOS | ||
| BIOGEN | 157,65 | -1,75 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,770 | +2,97 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,830 | -0,35 % | HEIDELBERG PHARMA AG unter Beobachtung |
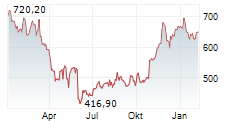
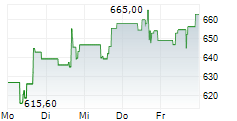
REGENERON PHARMACEUTICALS INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +0,58 % bei einem Aktienkurs von 663,60 Euro. Aus der Ausschüttung der letzten 12 Monate von insgesamt 3,02 Euro errechnet sich eine Dividendendrendite von +0,46 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu REGENERON PHARMACEUTICALS INC finden Sie auf Wallstreet Online.