- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 16.01. | ArriVent stock rating reiterated as Overweight by Cantor Fitzgerald | 5 | Investing.com | ||
| 16.01. | Cantor Fitzgerald bestätigt "Overweight"-Rating für ArriVent | - | Investing.com Deutsch | ||
| 06.01. | ArriVent BioPharma: Oppenheimer bestätigt "Outperform"-Rating und Kursziel von 44 US-Dollar | 3 | Investing.com Deutsch | ||
| ARRIVENT BIOPHARMA Aktie jetzt für 0€ handeln | |||||
| 06.01. | Oppenheimer reiterates Outperform rating on ArriVent BioPharma stock | 1 | Investing.com | ||
| 22.12.25 | Cantor Fitzgerald initiates ArriVent BioPharma stock with Overweight rating | 3 | Investing.com | ||
| 22.12.25 | ArriVent doses first patient in pivotal phase 3 trial for lung cancer drug | 4 | Investing.com | ||
| 22.12.25 | ArriVent BioPharma, Inc.: ArriVent Announces First Patient Dosed in Global Pivotal Phase 3 ALPACCA Trial Evaluating Firmonertinib for First-Line Treatment of EGFR PACC Mutant Non-Small Cell Lung Cancer | 238 | GlobeNewswire (Europe) | Firmonertinib has the potential to redefine first-line treatment in this underserved population as a once daily, oral, brain-penetrant, chemo-free monotherapyALPACCA pivotal trial is designed to support... ► Artikel lesen | |
| 10.12.25 | ArriVent BioPharma: BTIG startet mit Kaufempfehlung wegen Onkologie-Potenzial | 2 | Investing.com Deutsch | ||
| 10.12.25 | ArriVent BioPharma stock initiated with Buy rating at BTIG on oncology potential | 1 | Investing.com | ||
| 25.11.25 | ArriVent BioPharma stock initiated with Buy rating at Truist on lung cancer drug | 3 | Investing.com | ||
| 10.11.25 | ArriVent BioPharma stock price target raised to $47 from $32 at Clear Street | 5 | Investing.com | ||
| 10.11.25 | ArriVent BioPharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 10.11.25 | ArriVent BioPharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 22.09.25 | ArriVent BioPharma appoints Brent Rice as chief commercial officer | 2 | Investing.com | ||
| 22.09.25 | ArriVent BioPharma beruft Brent Rice zum Chief Commercial Officer | 1 | Investing.com Deutsch | ||
| 22.09.25 | ArriVent BioPharma, Inc.: ArriVent Appoints Brent S. Rice as Chief Commercial Officer | 355 | GlobeNewswire (Europe) | NEWTOWN SQUARE, Pa., Sept. 22, 2025 (GLOBE NEWSWIRE) -- ArriVent BioPharma, Inc. (Company or ArriVent) (Nasdaq: AVBP), a clinical-stage company dedicated to accelerating the global development of... ► Artikel lesen | |
| 09.09.25 | ArriVent reports 16-month PFS for lung cancer drug firmonertinib | 1 | Investing.com | ||
| 09.09.25 | ArriVent BioPharma, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 11.08.25 | ArriVent BioPharma, Inc.: ArriVent BioPharma Reports Second Quarter 2025 Financial Results | 969 | GlobeNewswire (Europe) | Positive interim Phase 1b update underscores firmonertinib's potential in EGFR PACC mutant NSCLC; global pivotal Phase 3 ALPACCA study expected to enroll first patient in 2H 2025Dosed the first patient... ► Artikel lesen | |
| 21.07.25 | ArriVent BioPharma, Inc.: ArriVent's Topline Pivotal Phase 3 FURVENT Data for Firmonertinib in First-Line NSCLC EGFR Exon20 Insertion Mutations is Projected to be Early 2026 | 523 | GlobeNewswire (Europe) | Enrollment in FURVENT was completed in Q1 2025 Firmonertinib received FDA Breakthrough Therapy Designation in this patient population NEWTOWN SQUARE, Pa., July 21, 2025 (GLOBE NEWSWIRE) -- ArriVent... ► Artikel lesen |
1 2 Weiter >>
21 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| ERASCA | 15,120 | +5,04 % | Erasca, Inc. (ERAS): A Bull Case Theory | ||
| QIAGEN | 40,875 | +0,43 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| DYNE THERAPEUTICS | 15,230 | -5,67 % | Dyne Therapeutics, Inc.: Dyne Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Business Highlights | - Planned submission for U.S. Accelerated Approval of z-rostudirsen on track for Q2 2026; potential launch in Q1 2027 - - Positive topline results reported from Phase 1/2 DELIVER trial of z-rostudirsen... ► Artikel lesen | |
| BIONTECH | 89,10 | -0,11 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| DAY ONE BIOPHARMACEUTICALS | 12,865 | 0,00 % | Day One BioPharmaceuticals gains amid takeover speculation | ||
| ADMA BIOLOGICS | 16,545 | -0,21 % | ADMA Biologics Announces $200 Mln Capital Return Plan | ||
| MODERNA | 49,585 | -0,17 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 86,15 | -4,39 % | Kymera auf TD Cowen Konferenz: Strategische Einblicke in Medikamenten-Pipeline und Partnerschaften | ||
| EVOTEC | 5,652 | +6,56 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| ARCELLX | 114,26 | +0,18 % | Weekly Buzz: MGNX's LINNET Trial On Hold; Eton Pharmaceuticals, ALUR Get FDA Nod; GILD Snaps Up ACLX | FOSTER CITY (dpa-AFX) - This week, the biotech space witnessed significant milestones, including FDA approvals, oncology drug acquisitions, and collaborations. Positive clinical trial results... ► Artikel lesen | |
| TYRA BIOSCIENCES | 35,240 | 0,00 % | Tyra Biosciences Reports Fourth Quarter and Full-Year 2025 Financial Results and Recent Highlights | - Launched "dabogratinib 3x3" strategy to pursue 3 late-stage clinical studies in LG-UTUC, IR NMIBC and ACH, 3 potential blockbuster indications -
- Interim Ph2... ► Artikel lesen | |
| SANA BIOTECHNOLOGY | 3,820 | 0,00 % | Sana Biotechnology, Inc: Sana Biotechnology Reports Fourth Quarter and Full Year 2025 Financial Results and Business Updates | Shared 12-month clinical results of ongoing UP421 type 1 diabetes study showing that hypoimmune-modified pancreatic islet cells transplanted without immunosuppression are safe and well-tolerated,... ► Artikel lesen | |
| CG ONCOLOGY | 61,50 | 0,00 % | CG Oncology Inc.: CG Oncology Reports 2025 Year End Financial Results and Provides Business Updates | PIVOT-006 Phase 3 topline data evaluating cretostimogene monotherapy as an adjuvant therapy in intermediate-risk NMIBC expected first half 2026CORE-008 Cohort CX Phase 2 first results of combination... ► Artikel lesen | |
| TECTONIC THERAPEUTIC | 27,410 | +19,17 % | Tectonic Therapeutic Announces Fourth Quarter and Full Year 2025 Financial Results and Recent Business Highlights | Announced positive topline results from TX45 Phase 1b acute hemodynamic clinical trial in patients with Group 2 Pulmonary Hypertension in Heart Failure with reduced Ejection Fraction ("PH-HFrEF")... ► Artikel lesen | |
| UPSTREAM BIO | 7,800 | +1,56 % | Upstream Bio Presents Additional Analyses from the Phase 2 VIBRANT Trial of Verekitug in Chronic Rhinosinusitis with Nasal Polyps at 2026 AAAAI Annual Meeting | - Primary endpoint of endoscopic nasal polyp score (NPS) showed reduction of -1.95 (p in new analysis with adjustment for concomitant rescue therapy use - - Secondary endpoints also provided strong... ► Artikel lesen |
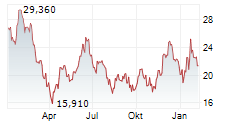
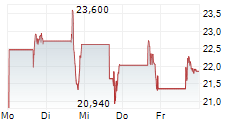
ARRIVENT BIOPHARMA INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 26,590 Euro und damit +14,71 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ARRIVENT BIOPHARMA INC finden Sie auf Wallstreet Online.