GAITHERSBURG, Md., April 28, 2014 /PRNewswire/ --A rigorous multi-center clinical trial has completed testing over 400 diabetic patients to evaluate the effectiveness of RETeval® based visual electrophysiology in assessing sight threatening diabetic retinopathy (DR) in comparison to the gold standard of dilated seven field stereo fundus photography, double read and adjudicated.
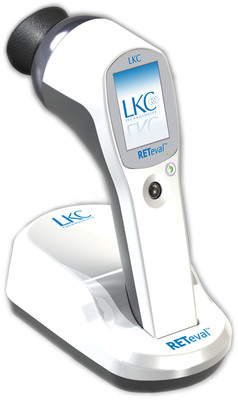
RETeval®, a handheld DR screening device, enables any health care provider to screen a diabetic for sight-threatening DR in less than three minutes with no dilation - regardless of pupil size or the presence of cataracts.
"The preliminary results are excellent," said LKC Technologies President James J. Datovech. "The medical, diabetic care and public health communities will find great value in this technology." Visit LKC at the LKC Technologies' booth (#300) at the ARVO Annual Meeting in Orlando, Florida, May 4-7.
The RETeval® clinical trial Primary Investigator is Stephen R. Fransen, Associate Professor at the Dean McGee Eye Institute, University of Oklahoma and Chief Medical Officer of Inoveon, a provider of ETDRS 7-field stereo fundus photography services.
According to Mr. Datovech, "This brings DR screening to wherever diabetics meet their care, enabling them to refer at risk diabetics to ophthalmologists that manage diabetic retinopathy and CSME." He continued,"Using skin electrodes (not corneal electrodes) and a simple handheld device, minimizes the cost and complexity of the DR screening equation."
Announcement: LKC also announces a new RETeval® option at ARVO allowing RETeval® to be a full function, hand-held, flash ERG/VEP device that will run the complete five and/or six step ISCEV flash ERG protocol using skin electrodes.
"This fast, efficient, cost effective, easy to use device provides the capability for physician offices, hospitals, clinics, research facilities and others around the globe to conduct visual electrophysiology," Mr. Datovech said.
RETeval® is CE marked and approved in Canada, Australia and Japan. It is in use in the U.K., India, Canada, Malaysia, and Japan.
This release contains forward-looking statements related to growth drivers, performance, market position, strategies for growth, and LKC's future operating results, which are subject to risks and uncertainties, including competitive factors, difficulties and delays inherent in the development, manufacturing, marketing and sale of medical products, government regulation and general economic conditions. Actual results may differ materially from anticipated results. LKC does not update its forward-looking statements.
Photo - http://photos.prnewswire.com/prnh/20131120/NY20067
SOURCE LKC Technologies, Inc.
