Chart-Typ:
Zeitraum:
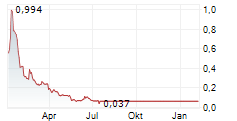
| Palatin Technologies, Inc.: Palatin Reports Second Quarter Fiscal Year 2026 Financial Results and Provides Corporate Update | Advancing differentiated MC4R-based obesity programs into clinical development
Melanocortin-based obesity therapies targeting rare MC4R pathway disorders with... ► Artikel lesen |
| Palatin Technologies, Inc.: Palatin Reports First Quarter Fiscal Year 2026 Financial Results and Provides Corporate Update | Significant progress on multiple fronts - obesity pipeline advancing towards the clinic, out-licensing collaboration, strengthened balance sheet, and reinstatement... ► Artikel lesen |
| Palatin Technologies, Inc.: Palatin Technologies Announces Closing of Upsized $18.2 Million Public Offering with the Full Exercise of the Underwriters' Over-Allotment Option | Trading of Palatin's common shares resumed trading on NYSE today, November 12, 2025, under the symbol "PTN"
PRINCETON, N.J., Nov. 12, 2025 /PRNewswire/ -- Palatin... ► Artikel lesen |
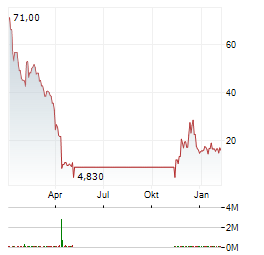
| 1CM Inc.: SNDL & 1CM Complete Purchase and Sale of 5 Retail Stores in Alberta and Saskatchewan | Edmonton, Alberta and Toronto, Ontario--(Newsfile Corp. - January 7, 2026) - SNDL Inc. (NASDAQ: SNDL) (CSE: SNDL) ("SNDL") and 1CM Inc. (CSE: EPIC) (OTCQB: MILFF) (FSE: IQ70) ("1CM") are pleased... ► Artikel lesen |
| 1CM Inc.: SNDL & 1CM Provide Update Regarding Arrangement | Edmonton, Alberta and Toronto, Ontario--(Newsfile Corp. - December 15, 2025) - SNDL Inc. (NASDAQ: SNDL) (CSE: SNDL) ("SNDL") and 1CM Inc. (CSE: EPIC) (OTCQB: MILFF) (FSE: IQ70) ("1CM") announce... ► Artikel lesen |
| SNDL Inc.: SNDL & 1CM Provide Update Regarding Arrangement | EDMONTON, Alberta and TORONTO, Dec. 15, 2025 (GLOBE NEWSWIRE) -- SNDL Inc. (Nasdaq: SNDL, CSE: SNDL) ("SNDL") and 1CM Inc. (CSE: EPIC) (OTCQB: MILFF) (FSE: IQ70) ("1CM") announce that they have entered... ► Artikel lesen |
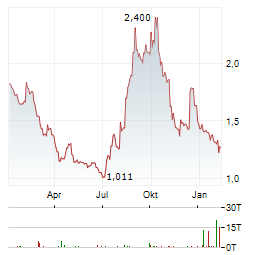
| Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Uplisting from Nasdaq Capital Market to Nasdaq Global Select Market | BERKELEY HEIGHTS, N.J., March 03, 2026 (GLOBE NEWSWIRE) -- Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) ("Tonix" or the "Company"), a fully integrated, commercial biotechnology company, today... ► Artikel lesen |
| Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Program Updates on Phase 2/3-Ready Long-Acting Monoclonal Antibody (mAb) Designed for Seasonal Prevention of Lyme Disease (TNX-4800) | Exploring clinical development plan options including a controlled human infection model (CHIM) and a Phase 2/3 adaptive field study Expect to have investigational product of TNX-4800 (anti-Borrelia... ► Artikel lesen |
| Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Licensing TNX-4900, a Selective Sigma-1 Receptor Antagonist for Chronic Neuropathic Pain from Rutgers University | Non-opioid analgesic shows efficacy in several animal pain models, including diabetic and chemotherapy-induced neuropathic pain Compelling safety and pharmacokinetic profiles in animals support IND-enabling... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CROWN LNG HOLDINGS LIMITED finden Sie auf Wallstreet Online.