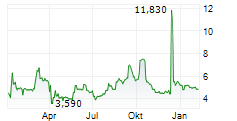
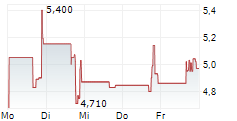
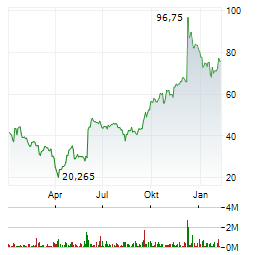
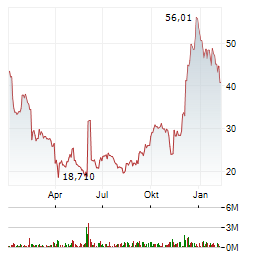
Chart-Typ:
Zeitraum:
| Kymera Therapeutics, Inc.: Kymera Therapeutics Announces First Patient Dosed in BREADTH Phase 2b Asthma Clinical Trial of KT-621, a First-in-Class, Oral STAT6 Degrader | Data from the BREADTH Phase 2b asthma trial is expected to be reported in late-2027 Data from the ongoing parallel BROADEN2 Phase 2b atopic dermatitis trial is expected to be reported by mid-2027... ► Artikel lesen |
| Kymera Therapeutics, Inc.: Kymera Therapeutics Outlines Key 2026 Objectives and Strategy to Advance Industry Leading Portfolio of Oral Immunology Programs | KT-621 BROADEN2 Phase 2b trial in AD ongoing, with data expected by mid-2027 KT-621 BREADTH Phase 2b trial in asthma initiated, with data expected in late-2027 KT-579 Phase 1 HV clinical trial expected... ► Artikel lesen |
| Kymera Therapeutics, Inc.: Kymera Therapeutics Announces Closing of Upsized $602 Million Public Offering and Full Exercise of Underwriters' Option to Purchase Additional Shares | WATERTOWN, Mass., Dec. 11, 2025 (GLOBE NEWSWIRE) -- Kymera Therapeutics, Inc. (NASDAQ: KYMR), a clinical-stage biopharmaceutical company advancing a new class of oral small molecule degrader medicines... ► Artikel lesen |
| Vera Therapeutics Announces Appointment of Matt Skelton to Chief Commercial Officer | BRISBANE, Calif., Jan. 28, 2026 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. (Nasdaq: VERA), a late clinical-stage biotechnology company focused on developing and commercializing transformative treatments... ► Artikel lesen |
| Vera Therapeutics Announces U.S. FDA Granted Priority Review to Biologics License Application for Atacicept for Treatment of Adults with IgA Nephropathy | FDA assigned PDUFA target action date of July 7, 2026.If approved, atacicept would be the first B cell modulator targeting both BAFF and APRIL for IgAN.Atacicept received FDA Breakthrough Therapy... ► Artikel lesen |
| Vera Therapeutics Submits Biologics License Application to U.S. FDA through Accelerated Approval Program for Atacicept for the Treatment of Adults with IgA Nephropathy | Atacicept received FDA Breakthrough Therapy Designation for the treatment of IgANORIGIN Phase 3 trial met its primary endpoint at the prespecified interim analysis of proteinuria reduction with 46%... ► Artikel lesen |
| Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu RADIOPHARM THERANOSTICS LIMITED ADR finden Sie auf Wallstreet Online.