LumiThera and Product Creation Studio Honored for Product Design
SEATTLE, June 12, 2019 /PRNewswire/ -- LumiThera, Inc. and Product Creation Studio today announced that LumiThera's Valeda Light Delivery System has been honored with a silver award in the 21st Annual Medical Design Excellence Awards competition. The 2019 winners were announced at the MDEA Ceremony on Tuesday, June 11, 2019 in conjunction with the MD&M East event in New York, New York.
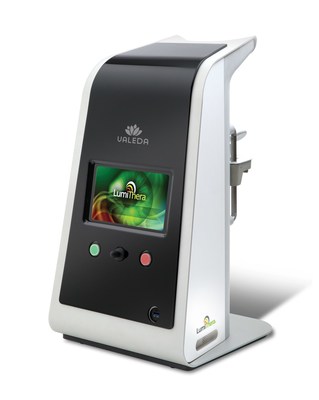
LumiThera, Inc. is a leader in the use of photobiomodulation for the treatment of acute and chronic ocular diseases and disorders. Age-related macular degeneration (AMD) is the leading cause of blindness in adults over 65. Product Creation Studio worked with LumiThera from product concept through transfer to manufacturing to design and engineer a medical device that would accurately, safely, and effectively treat patients suffering from dry AMD. The resulting Valeda Light Delivery System is currently the only approved treatment using Photobiomodulation for dry age-related macular degeneration in the European Union.
The MDEA are the medtech industry's premier design competition committed to recognizing significant achievements in medical product design and engineering that improve the quality of healthcare delivery and accessibility. The awards program celebrates the accomplishments of the medical device manufacturers, their suppliers, and the many people behind the scenes-engineers, scientists, designers, and clinicians-who are responsible for the cutting-edge products that are saving lives; improving patient healthcare; and transforming medtech-one innovation at a time.
"This award recognizes the efforts of the LumiThera and the Product Creation teams who have worked diligently to deliver a life changing treatment for those suffering from dry AMD," said Clark Tedford, PhD, CEO and President. "We are pleased to bring the Valeda Light Delivery System to the ophthalmic market and offer dry AMD patients a treatment for this debilitating disease."
"Product Creation Studio's collaboration with LumiThera embodies what our organization is about: empowering innovators with the right design and development services at the right time to deliver products with impact," Scott Thielman, Chief Technology officer of Product Creation Studio, said. "The Valeda Light Delivery System is a breakthrough product that makes a meaningful difference for patients and their families. It has been an honor to support the LumiThera team with comprehensive engineering and design services from the company's early days through production."
The 2019 MDEA Juror Panel selected 44 exceptional finalists in nine medical technology product categories. Products were judged based on design and engineering innovation; function and user-related innovation; patient benefits; business benefits; and overall benefit to the healthcare system. Unlike other design competitions that are merely styling contests, the MDEA jury is comprised of a balance of practicing doctors, nurses, and technicians alongside industrial designers, engineers, manufacturers, and human factors experts.
About LumiThera
LumiThera, Inc. is a commercial-stage medical device company focused on treating people affected by ocular disorders and diseases including dry age-related macular degeneration, a leading cause of blindness in adults over 65. The company is a leader in the use of PBM for treatment of acute and chronic ocular diseases and disorders. The company is developing the office-based Valeda Light Delivery System to be used by eye care specialists as medical treatments.
The Valeda Light Delivery System has been granted authorization to use the CE Mark by an EU Notified Body as required for commercial use in the European Union only. Valeda is not approved for use by the Food & Drug Administration (FDA) in the USA.
About Product Creation Studio
Product Creation Studio empowers organizations to break through barriers in new product development. With a design-centered approach and expertise in developing consumer, medical, and industrial products, our multi-disciplinary team is a force multiplier that provides all the skills needed to bring a product vision to reality. Our ISO 13485:2016-certified quality system, PCS+, ensures quality development standards are applied to each and every project. From early funding to late stage development, we supercharge development efforts, reduce time to market, and create exceptional user experiences for increased market success. Contact us to make your product vision a reality.
Photo - https://mma.prnewswire.com/media/901294/LumiThera_Inc_Valeda.jpg

