COMPANY WILL POST INITIAL FORECASTS FOR EACH GENACCORD! PRODUCT JUST AS SOON AS FORECAST AND AVAILABILITY DISCUSSIONS ARE COMPLETED WITH OUR TAIWANESE PARTNER
LOS ANGELES, CA / ACCESSWIRE / January 21, 2019 / Decision Diagnostics Corp. (OTC PINK:DECN) is an 18-year old, diabetes-focused bio-technology development firm, manufacturer, quality plan administrator, FDA registered medical device customer support organization, and exclusive worldwide sales and regulatory process agent for the GenUltimate! ("Sunshine") diabetes test strip, its GenAccord! systems for the uninsured and under-insured, its GenChoice! ("Ladybug") test strip now in the later stages of FDA 510(k) prosecution, and its GenUltimate! Sure and GenUltimate Precis products manufactured for International markets. The company also markets its PetSure! test strip for the diabetic testing of dogs and cats, a diagnostic specifically designed to run on the market leading Zoetis Alpha Trak meter system as well as the GenUltimate! 4Pets Test strip and Avantage! meter. Finally the company has completed design and testing of its GenUltimate! TBG ("Dragonfly") diabetes testing system, now coming up on a clinical trial slot in Korea, license to our strategic partner, and FDA 510(k) clearance.
In addition, today DECN announces that what began with the initial acceptance of a gesture from our proposed strategic licensing partner to add our GenAccord! meter and test strips to our upcoming agreement, has evolved into much more. The company has completed its market evaluation and targeting and is introducing its FDA 510(k) cleared GenAccord! Enhanced product line into retail, charity and direct distribution markets that serve the uninsured and under-insured diabetics, which number approximately 12 million in the U.S. alone. GenAccord! Enhanced is also going to be independently targeted to the EU, India and Russian Federation markets, which taken together are 70% of the size of the domestic U.S. market.
Keith Berman, CEO of DECN commented, "We have completed the packaging for our GenAccord! products and plan introduction in the USA of GenAccord! Enhanced just as soon as product arrives in our warehouses, either in late January or early February 2020. Sometime in early 2Q 2020 we will introduce GenAccord! Enhanced into the EU markets and shortly thereafter into the Russian Federation through our strategic partner there."
The company also announced that the FDA 510(k) cleared GenAccord! Rely MX will be launched in late 1Q 2020 to service the needs of small hospitals, long term care facilities and clinics, where a multi-patient meter is required. GenAccord! Rely MX is specifically designed for the needs of these markets where there is lesser competition and higher profit margins.
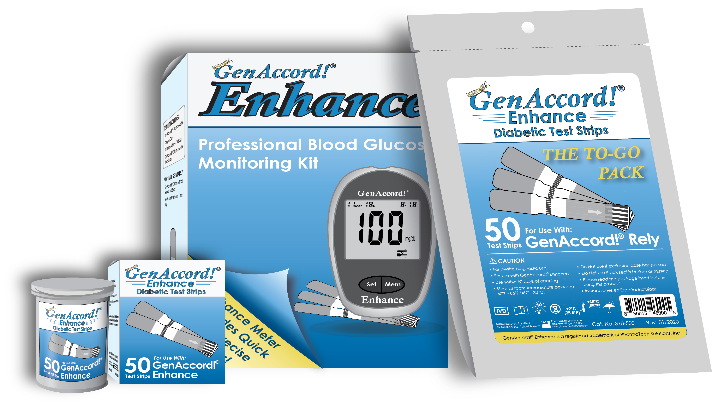

Mr. Berman concluded, "The GenAccord! Enhance meter will retail for $8.95, a second meter purchased at the same time, to be used at the diabetic's work or school will be bundled for an additional $2.95. A vial of 50 test strips will retail for $7.95, and a package of 50 test strips which are individually wrapped will retail for $8.79. We have labeled the package of 50 individually wrapped test strips our "To-Go Pack," referring to a diabetic's ability to take a day's testing needs without carrying around a vial of strips. The "To-Go Pack" is novel because the strips can be stored at home, at work, in a car, or luggage, and remain fresh for two years. When a vial of test strips is opened by a diabetic, the strips immediately become exposed to the air, and are only good for use for 90 days. The "To-Go Pack" offers up to 24 months of stable reliable testing.
ABOUT DECISION DIAGNOSTICS CORP
Decision Diagnostics Corp. is the leading manufacturer and worldwide distributor of diabetic test strips engineered to operate on legacy glucose meters. DECN's products are designed to operate efficiently and less expensively on certain glucose meters already in use by almost 7.5 million diabetics worldwide. With new inspired technology diabetic test strips already in the final stages of development, DECN products compete on a worldwide scale with legacy manufacturers currently selling to 71+ percent of a $15+ billion at-home testing market. The company's GenUltimate TBG product is not yet available for sale in the United States or Puerto Rico but is expected to go on sale in select International markets in February 2020.
Forward-Looking Statements:
This release contains the company's forward-looking statements which are based on management's current expectations and assumptions as of January 20, 2019 regarding the company's business and performance, its prospects, current factors, the economy, and other future conditions and forecasts of future events, circumstances, and results.
CONTACT INFORMATION:
Decision Diagnostics Corp.
Keith Berman
(805) 446-2973
info@decisiondiagnostics.co
www.genultimate.com
www.genultimatetbg.com
www.petsureteststrips.com
www.pharmatechdirect.com
SOURCE: Decision Diagnostics Corp.
View source version on accesswire.com:
https://www.accesswire.com/573634/DECN-adds-to-Domestic-and-International-Channels-for-its-GenAccord-Product-Inclusive-of-the-EU-and-Russian-Federation-as-Company-also-Schedules-Entry-of-2nd-GenAccord-Product-for-Multi-Patient-Use
