SHANGHAI, March 19, 2020 /PRNewswire/ -- Recently, scientists at Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences, together with scientists at Institute of Biophysics (IBP) of Chinese Academy of Sciences and Monash University, determined two cryo-electron microscopy (cryo-EM) structures of the human glucagon receptor (GCGR) in complex with glucagon and distinct classes of G proteins, Gs or Gi. The study will be published in Science on Mar. 20, 2020.
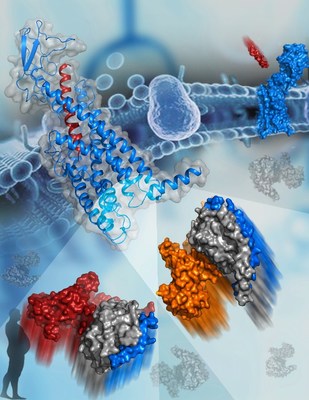
G protein-coupled receptors (GPCRs) play essential roles in cell signal transduction, and serve as important therapeutic targets for a large number of diseases. Upon binding to extracellular agonists, GPCRs stimulate various signaling pathways by recruiting different G proteins (Gs, Gi, and Gq, etc.) to mediate a wide variety of physiological functions. The selective coupling between a GPCR and specific G proteins is critical for the biological action of the receptor.
However, the molecular details that define how an individual GPCR recognizes different G protein subtypes remain elusive, thus limiting the understanding of mechanisms of GPCR signal transduction.
This study offers valuable insights into pleiotropic GPCR-G protein coupling and G protein specificity. The most exciting finding is that the sixth transmembrane helix (helix VI) of GCGR adopts a similar outward shift in the two G protein-bound GCGR structures, forming a common binding cavity to accommodate Gs and Gi. This common G protein binding pocket is consistent with the signaling pleiotropy of GCGR, and allows for maximal efficiency in activating various pathways.
Although GCGR couples to both G proteins through the common pocket, it does so with different interaction patterns, which account for G protein specificity. The measured interaction interface between GCGR and Gs is much larger than for Gi, resulting in higher binding affinity of Gs to the receptor. This offers a structural basis for the preferential coupling of GCGR to Gs.
Based on the structures of GCGR-G protein complexes, the researchers performed extensive functional studies using techniques such as mutagenesis, G protein activation and cell signaling to investigate the roles of key residues in the receptor-G protein binding interface in Gs and Gi activation. The results show that conformational differences of intracellular loops and residue side chains in the receptor are sufficient to guide G protein selectivity.
These findings present new opportunities for drug discovery by designing biased ligands to selectively block one specific signaling pathway, thus resulting in reduced side effects.
Photo - https://mma.prnewswire.com/media/1134716/cryo_EM_structures_GCGR.jpg

