Growing need for visibility and speed driving shift to digital regulatory systems
BARCELONA, Spain, Feb. 28, 2023 /PRNewswire/ -- New research shows a majority of medtech organizations are taking action to advance regulatory affairs, according to the second annual Veeva MedTech Regulatory Benchmark Report. More than half of medtech companies say establishing a single source for regulatory information (62%) and implementing a global and centralized regulatory information management (RIM) system (51%) will be the industry's top focus over the next two years. With the move to modernize RIM underway, medtech leaders are bringing together data, content, and systems for improved insights and time to market.
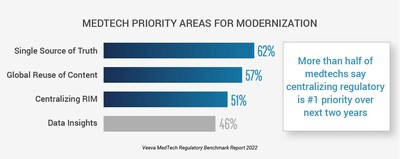
Data reveals an increased focus on digital systems to harmonize operations globally, with two out of every five companies already selecting digital regulatory technology. This shift toward digital RIM systems highlights the need for greater transparency and collaboration across regional teams to meet evolving regulations and change management requirements.
The report reveals positive changes to regulatory affairs and opportunities for improvement, including:
- Manual processes still linger: Just 13% of companies have implemented a global digital RIM system for end-to-end management of regulatory operations. Many medtech organizations rely on manual processes, disconnected data sources, and siloed systems that aren't scalable, making it harder to expand into new markets.
- Single source for registration data trends upward: Companies who collect global registration data in a single system increased year-over-year, highlighting urgency for establishing one source for regulatory information.
- Post-market compliance processes lack connections: Most companies (83%) have partial integrations, point solutions, or manual processes across regulatory and quality to capture post-market changes within the product portfolio. The lack of a seamless and automated change control process increases risks and can lead to compliance issues or delays.
"With new regulations and increasing supply chain complexity, medtech companies are evaluating paths to more seamless processes so high-quality data can be shared across teams," said Seth Goldenberg, vice president, Veeva MedTech. "This research shows the medtech industry is making progress by advancing regulatory operations for better global visibility, data accuracy, and compliance."
The Veeva MedTech Regulatory Benchmark Report examines the medical device and diagnostic industry's progress toward modernizing regulatory operations. Survey respondents include regulatory affairs professionals from more than 100 medtech organizations around the globe, ranging from enterprise to midsize businesses. See the full annual study which explores how medtech companies manage global compliance and visibility, speed to market, post-market compliance, and modernization.
About Veeva Systems
Veeva (NYSE: VEEV) is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world's largest pharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com/eu.
Contact: | |
|
|

Photo - https://mma.prnewswire.com/media/2011266/Veeva_Systems_Regulatory_Benchmark_Report_charts_Modernization_Priorities.jpg
Logo - https://mma.prnewswire.com/media/1488285/Veeva_Systems_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/industrywide-survey-reveals-centralizing-regulatory-is-number-one-priority-for-medtech-301757550.html
View original content:https://www.prnewswire.co.uk/news-releases/industrywide-survey-reveals-centralizing-regulatory-is-number-one-priority-for-medtech-301757550.html

