HAMBURG, Germany, July 13, 2023 /PRNewswire/ -- Olympus Corporation, a global medical technology company, announced publication of study data demonstrating that the minimally invasive iTind treatment provides long-lasting relief of more than four years for people suffering from the symptoms of an enlarged prostate, also known as BPH.
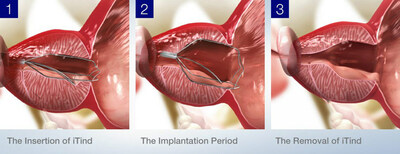
The long-term study data demonstrates that the iTind procedure leads to significant and durable reduction of BPH-related LUTS (lower urinary tract symptoms) and improved IPSS (International Prostate Symptom Score) and QoL (Quality of Life) for over 50 months and up to 79 months (6.6 years) following treatment.1
Due to the COVID-19 pandemic, patients could not be examined in-person for functional testing at the >48-month follow-up. Hence, the protocol was adapted so that data regarding long-term symptoms' relief, (IPSS), QoL improvement and the need for re-treatment could be gathered telephonically.
Summary of Long-term Study Results
- Fifty patients pursued the prospective, single-arm, multicenter study beyond 36 months following treatment. The study analyzes results for 41 of the 50 patients beyond 48 months. Nine of the 50 were unavailable for follow-up: five patients were lost to follow-up; two patients died unrelated to iTind device placement; and two patients (36-48 months follow-up) required surgical re-treatments (one transurethral resection of prostate, one thulium laser enucleation of prostate).
- iTind device treatment showed significant improvement in symptoms, with IPSS reduction of 45.3% and IPSS-QoL reduction of 45.1% from baseline up to 79 months post-procedure (both P<0.0001)
- No late post-operative complications were reported beyond 36 months of follow-up, and no patients required additional medication.
- The surgical re-treatment rate after 36 months was 4%, and the total cumulative re-treatment rate from baseline up to 79 months was 11.1%.
The study was funded by Medi-Tate, a wholly owned subsidiary of Olympus Corporation.
To read the full press release, visit: www.olympus-europa.com
About Olympus
For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit www.olympus-europa.com.
1 | Amparore D, De Cillis S, Schulman C, Kadner G, Fiori C, Porpiglia F. Temporary implantable nitinol device for benign prostatic hyperplasia-related lower urinary tract symptoms: over 48-month results [published online ahead of print, 2023 Jun 23]. Minerva Urol Nephrol. 2023;10.23736/S2724-6051.23.05322-3. doi:10.23736/S2724-6051.23.05322-3 |

Photo - https://mma.prnewswire.com/media/2152729/Olympus_Corporation_itind_threesteps.jpg
Logo - https://mma.prnewswire.com/media/1774929/4160711/Olympus_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/study-proves-itind-treatment-for-enlarged-prostate-lasts-more-than-four-years-301876989.html
View original content:https://www.prnewswire.co.uk/news-releases/study-proves-itind-treatment-for-enlarged-prostate-lasts-more-than-four-years-301876989.html

