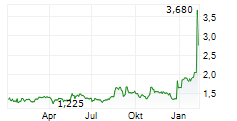
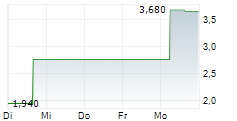
DJ Affluent Medical announces several key appointments for Medical and Clinical affairs.
Affluent Medical
Affluent Medical announces several key appointments for Medical and Clinical affairs.
11-March-2025 / 17:45 CET/CEST
Dissemination of a French Regulatory News, transmitted by EQS Group.
The issuer is solely responsible for the content of this announcement.
=----------------------------------------------------------------------------------------------------------------------
PRESS RELEASE
Affluent Medical announces several key appointments
for Medical and Clinical affairs.
-- Appointment of Dr. Howard C. Herrmann as strategic Chief Medical Officer for structural heart platform.
-- Appointment of Pr. Nicolas Barry Delongchamps as strategic Chief Medical Officer for Urology platform.
-- Federica Azzimonti joins as Director of Clinical Operations, bringing extensive expertise to optimize
clinical trial readiness for market launch.
Aix-en-Provence, March 11, 2025 - 5:45 p.m. CET - Affluent Medical (ISIN: FR0013333077 - Ticker: AFME - "Affluent"), a
French clinical-stage medical technology company specializing in the development and industrialization of implantable
innovative medical devices, today announced three key leadership appointments. These strategic additions aim to
accelerate the company's progress towards market access for its advanced product pipeline.
New Chief Medical Officers to Drive Specialized Platforms
Affluent Medical has appointed Dr. Howard C. Herrmann as strategic Chief Medical Officer (CMO) for its Structural Heart
Platform and Pr. Nicolas Barry Delongchamps as strategic CMO for its Urology Platform. These key appointments
underscore Affluent Medical commitment to enhancing its clinical expertise and driving innovation as it advances its
groundbreaking devices, including mitral ring KALIOSTM, mitral valve EPYGON and artificial urinary sphincter ARTUS.
Dr. Howard C. Herrmann, a renowned interventional cardiologist, is Professor of Medicine at the Perelman School of
Medicine at the University of Pennsylvania and Director of Interventional Cardiology at the Hospital of the University
of Pennsylvania, Philadelphia. With over three decades of experience in transcatheter device therapies for valvular and
structural heart diseases, he has authored over 500 publications and served as President of the Pennsylvania Chapter of
the American College of Cardiology. His profound expertise in structural heart care will guide the development of
Affluent's next-generation structural heart devices.
Pr. Nicolas Barry Delongchamps, a distinguished urologist and professor at Paris-Cité University, brings extensive
expertise in minimally invasive surgery for benign prostatic hyperplasia and urinary incontinence. His knowledge and
experience with urinary incontinence will support Affluent Medical's strategy in urology, driving innovation in this
critical area.
Expanding Operational Expertise with New Director of Clinical Operations
Further bolstering its operational capabilities, Affluent Medical has named Federica Azzimonti as Director of Clinical
Operations. With close to 25 years of experience in managing international clinical studies across cardiovascular and
other medical specialties, Federica brings a wealth of expertise in trial optimization and execution. In collaboration
with both strategic CMOs, she will lead the operational aspects of Affluent Medical clinical trials, ensuring
streamlined execution as the company prepares for pivotal stages.
"These appointments come at a crucial time as we enter our next phase of growth, transitioning into pivotal phases and
continuing to progress towards commercialization of our devices." said Sébastien Ladet, CEO of Affluent Medical. "The
addition of strong medical experts has been a deliberate move that demonstrates Affluent Medical commitment to execute
and to offer the best available therapies worldwide."
About Affluent Medical's Specialized Platforms
MITRAL RING KALIOSTM:
KaliosTM is the only mitral annuloplasty device that can be simply adjusted percutaneously by a cardiologist to treat
both residual or recurrent mitral valve insufficiency, at any time after implantation, repeatedly and with a beating
heart, thereby avoiding repeat open-heart surgery. Affluent Medical believes that KaliosTM would avoid further
intervention for potentially 30% to 40% of patients over a five-year horizon.
Following positive feedback from the FDA in September 2024, Affluent Medical's objective is to submit a De Novo
application with current clinical data at the end of 2025/early 2026 to be followed by commercial launch, subject to
Edwards' decisions, with whom Affluent Medical has signed several agreements related to its structural heart products
(adjustable mitral annulus KaliosT) and technologies (mitral valve technology).
MITRAL VALVE EPYGON:
Epygon is the only biomimetic mitral heart valve that mimics the anatomy of the native mitral valve and physiological
blood flow, able to be implanted via a transcatheter route. This transcatheter approach avoids an invasive open-heart
procedure and associated complications to treat mitral valve insufficiency.
In 2024, the company began a collaboration with Prof. Mohammad Sarraf, MD, interventional cardiologist at the Mayo
Clinic in the US, to evaluate the benefits of the biomimetic design of the Epygon valve. This innovative design aims to
replicate the anatomy and natural physiology of the native mitral valve to enable patients to recover good cardiac
function more quickly.
During the first half of 2024, Affluent Medical accelerated patient screening, achieving a fourfold increase in the
number of patients screened by the end of June 2024 with the goal to implant up to ten patients to complete the pilot
phase.
URINARY SPHINCTER ARTUS:
Artus is the first artificial urinary sphincter that can be activated by the patient with a simple remote control for
the treatment of moderate to severe urinary incontinence. Urinary incontinence is a major public health problem for
over 400 million people worldwide without any innovation in the last 40 years, causing patients to suffer a reduced
quality of life associated with the psychological disorders related to the disease.
In January 2025, the Company announced the completion of the enrollment for the pilot phase of the European multicenter
clinical study in humans with the successful 10th minimally invasive implantation of the Artus urinary sphincter with,
to date, 100% of devices having been successfully activated and the safety profile remains positive. The pivotal phase,
aiming to validate the device's performance in reducing incontinence in several dozen patients, is scheduled to begin
in Q2 2025.
With women representing about 80% of patients suffering from urinary incontinence, the Company will submit the dossier
in order to begin a pilot study in women in the first half of 2025, aiming to extend indications for its Artus urinary
sphincter.
About Affluent Medical
Affluent Medical is a French medical technologies company, founded by Truffle Capital, that aims to become a global
leader in the treatment of structural heart diseases, one of the world's leading causes of mortality, and urinary
incontinence, which currently affects one in four adults.
Affluent Medical develops next-generation implants that are minimally invasive, innovative, adjustable and biomimetic,
designed to restore essential physiological functions. The candidate products developed by the Company are all
undergoing clinical studies in humans.
Subject to raising the funds necessary to finance its strategy and the positive results of ongoing clinical studies,
the Company aims to gradually market its products from 2026, directly or indirectly.
For more information, please visit www.affluentmedical.com
Contacts:
SEITOSEI.ACTIFIN
AFFLUENT MEDICAL
Financial Communications / Press Relations
Ghislaine Gasparetto / Jennifer Jullia
Sébastien Ladet
+33 (0)6 21 10 49 24 / +33 (0)1 56 88 11 19
Chief Executive Officer
ghislaine.gasparetto@seitosei-actifin.com / jennifer.jullia@seitosei-actifin.com
investor@affluentmedical.com
MC SERVICES AG
PRIMATICE
Media Relations Europe
Public Relations France
Thomas Roborel de Climens Maximilian SCHUR / Julia BITTNER
+33 (0)6 78 12 97 95
+49 (0)211 529252 20 / +49 (0)211 529252 28
thomasdeclimens@primatice.com
affluent@mc-services.eu
-----------------------------------------------------------------------------------------------------------------------
Regulatory filing PDF file File: 20250311_PR_Affluent_Medical_CMOS_Director of Clinical Operations__vFinal
=------------------------------------------------------------------
Language: English
Company: Affluent Medical
320 avenue Archimède, Les pléiades III Bâtiment B
13100 Aix en Provence France
France
Phone: +33 4 42 95 12 20
E-mail: jerome.geoffroy@affluentmedical.com
Internet: https://www.affluentmedical.com/
ISIN: FR0013333077
Euronext Ticker: AFME
AMF Category: Inside information / Other releases
EQS News ID: 2098966
End of Announcement EQS News Service
=------------------------------------------------------------------------------------
2098966 11-March-2025 CET/CEST
Image link: https://eqs-cockpit.com/cgi-bin/fncls.ssp?fn=show_t_gif&application_id=2098966&application_name=news&site_id=dow_jones%7e%7e%7ef1066a31-ca00-4e1a-b0a4-374bd7d0face
(END) Dow Jones Newswires
March 11, 2025 12:46 ET (16:46 GMT)
© 2025 Dow Jones News