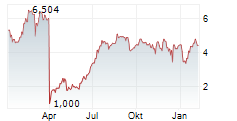
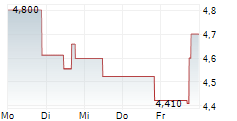
SAN FRANCISCO, April 10, 2025 /PRNewswire/ -- On April 3, 2025 investors in Aldeyra Therapeutics, Inc. (NASDAQ: ALDX) saw the price of their shares crash over 70% after the company announced that it received a Complete Response Letter ("CRL") from the FDA for the resubmission of the New Drug Application ("NDA") of reproxalap, an investigational drug candidate, for the treatment of dry eye disease.
This stark regulatory rejection, which contradicted Aldeyra's repeated assurances of imminent approval, erased over $200 million from the company's market capitalization, prompting investor rights law firm Hagens Berman to launch an investigation into potential securities law violations.
Hagens Berman urges investors who purchased Aldeyra shares and suffered substantial losses to submit your losses now. The firm also encourages persons with knowledge that may assist the firm's investigation to contact its attorneys.
Visit: www.hbsslaw.com/investor- fraud /aldx
Contact the Firm Now: [email protected]
844-916-0895
Aldeyra Therapeutics, Inc. (ALDX) Investigation:
The investigation is focused on the propriety of Aldeyra's statements about the prospects for FDA approval of the NDA of reproxalap.
In the past, Aldeyra touted the success of its Phase 3 dry eye clinical chamber trial for the drug and said the "results are uniquely supportive of the potential acute clinical effect of reproxalap on reducing ocular discomfort[.]" More recently, the company assured investors that "reproxalap is going to get approved on April 2 [.]"
Instead, on April 3, 2025, Aldeyra announced that it received the FDA's CRL. Aldeyra revealed that:
- "the FDA stated in the letter that the NDA 'failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes[;]'"
- "'at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye' should be conducted[;]" and
- "[t]he letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms."
In response to this news, the market swiftly reacted by sending the price of Aldeyra shares down over 70%, wiping out over $200 million of shareholder value.
Reed Kathrein, the Hagens Berman Partner leading the investigation, stated, "We are investigating whether Aldeyra may have misrepresented the propriety of its reproxalap study and results to investors."
If you invested in Aldeyra and have substantial losses, or have knowledge that may assist the firm's investigation, submit your losses now »
If you'd like more information and answers to frequently asked questions about the Aldeyra investigation, read more »
Whistleblowers: Persons with non-public information regarding Aldeyra should consider their options to help in the investigation or take advantage of the SEC Whistleblower program. Under the new program, whistleblowers who provide original information may receive rewards totaling up to 30 percent of any successful recovery made by the SEC. For more information, call Reed Kathrein at 844-916-0895 or email [email protected].
About Hagens Berman
Hagens Berman is a global plaintiffs' rights complex litigation firm focusing on corporate accountability. The firm is home to a robust practice and represents investors as well as whistleblowers, workers, consumers and others in cases achieving real results for those harmed by corporate negligence and other wrongdoings. Hagens Berman's team has secured more than $2.9 billion in this area of law. More about the firm and its successes can be found at hbsslaw.com. Follow the firm for updates and news at @ClassActionLaw.
SOURCE Hagens Berman Sobol Shapiro LLP