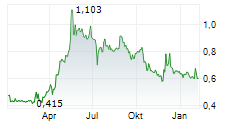
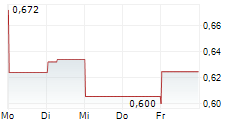
Cereno Scientific has successfully completed its planned Type C meeting with the FDA for lead asset CS1. The minutes from the meeting will be available after 30 days, but Cereno has indicated that the regulators looked to be in alignment with the Phase IIb study design and planned steps for clinical development. Note that unlike Type A and Type B meetings, Type C meetings are not strictly required, although they offer the opportunity to engage with the regulators to ensure alignment with study objectives, design and endpoints, which supports the likelihood of a successful outcome. Following receipt of the official minutes of the meeting and outcome of the subsequent IND, management plans to commence the Phase IIb trial in H126. As indicated in our previous note, we expect Cereno to self-sponsor Phase IIb, before seeking a licensing partner for the Phase III registrational study and subsequent commercialisation.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research