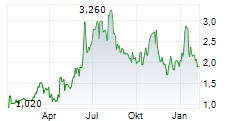
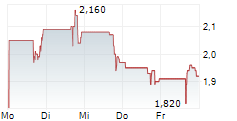
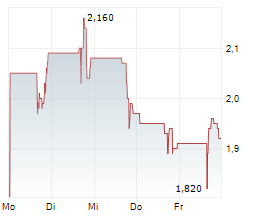
OKYO Pharma is advancing lead candidate urcosimod (previously OK-101) for neuropathic corneal pain (NCP), an area of unmet need with no approved treatment, as well as for inflammatory dry eye disease (DED). OKYO reported positive top-line data in early 2024 from its 240-patient Phase IIb study of urcosimod in patients with DED, showing improvements on both 'symptom' and 'clinical sign' endpoints. The company is engaging with the FDA to determine its next steps for urcosimod development in DED. Given the molecule's distinctive capability to also target pain, NCP may offer a further differentiated development pathway and this underscores OKYO's focus on the Phase IIb study in NCP.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research