- €15 million investment by Sanofi, in addition to the ongoing partnership including the development of BCMA targeting ANKET program in autoimmune indications
- First patient dosed in a Phase 1 study for IPH4502, Nectin-4 ADC in patients with selected advanced solid tumors. Presentations at AACR Annual Meeting 2025 and ASCO 2025 Annual Meeting
- FDA Breakthrough Therapy Designation granted to lacutamab for relapsed or refractory Sézary syndrome; long term follow up data from TELLOMAK Phase 2 to be presented at ASCO Annual Meeting 2025
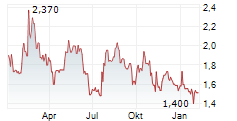
- Cash position of €72.5 million1 as of March 31, 2025, excluding the €15 million received from Sanofi, with a cash horizon to mid 2026
- Conference call to be held today at 2:00 p.m. CEST 8:00 a.m. ET
Regulatory News:
Innate Pharma SA (Euronext Paris: IPH; Nasdaq: IPHA) ("Innate" or the "Company") today reported its consolidated financial results for the quarter ending March 31, 2025.
"The first quarter of 2025 was marked by significant progress across both our pipeline and strategic partnerships," said Jonathan Dickinson, Chief Executive Officer of Innate Pharma. "Sanofi's €15 million equity investment reflects our shared conviction in the potential of our pipeline and the strength of our partnership. With the initiation of the Phase 1 study for our Nectin-4 ADC, IPH4502 and presentation at AACR and the FDA's Breakthrough Therapy Designation for lacutamab with upcoming ASCO presentations, we are well-positioned to deliver innovative therapies to patients. Our strengthened financial position provides a cash runway into mid 2026
Webcast and conference call will be held today at 2:00pm CEST (8:00am ET) Access to live webcast: https://events.q4inc.com/attendee/806006771 Participants may also join via telephone using the registration link below: https://registrations.events/direct/Q4I227404000000000000 This information can also be found on the Investors section of the Innate Pharma website, www.innate-pharma.com. A replay of the webcast will be available on the Company website for 90 days following the event. |
1 Including short term investments (€13.6m) and non-current financial instruments (€10.4m). |
Pipeline highlights
ANKET (Antibody-based NK cell Engager Therapeutics):
ANKET is Innate's proprietary platform for developing next-generation, multi-specific NK cell engagers to treat certain types of cancer.
IPH6501 (ANKET anti-CD20 with IL-2V, proprietary)
The Phase 1/2 clinical trial evaluating IPH6501 in B-cell Non-Hodgkin's lymphoma (B-NHL) is ongoing. The study is planned to enroll up to 184 patients. Clinical sites are open in the US, Australia and France and the first safety and preliminary activity data are expected in late 2025.
IPH6101 (ANKET anti-CD123)
In alignment with both companies' current strategic priorities, Sanofi and Innate agreed to terminate the 2016 Research Collaboration and Licence Agreement (the "2016 Agreement") as it relates to SAR'579/IPH6101 (CD123 ANKET); Innate will regain its rights on SAR'579/IPH6101 (CD123 ANKET
- The originally Sanofi-led Phase 1/2 study with SAR'579 IPH6101 (clinical study identifier: NCT05086315) is ongoing. Efficacy and safety results from the dose-escalation part, were shared in an oral presentation at the EHA 2024 Congress.
- In April 2024, Sanofi advanced SAR'579 IPH6101 to the Phase 2 preliminary dose expansion of the trial.
- The Parties will discuss a transition plan with regard to ongoing studies.
SAR'514/IPH6401, IPH62 (partnered with Sanofi)
SAR'514/IPH6401
As previously disclosed, Sanofi will opt to pursue the development of SAR'514/IPH6401 (BCMA ANKET) in autoimmune indications under the terms of the 2016 Agreement.
- The continued Sanofi-led Phase 1/2 study (clinical study identifier: NCT05839626) for the treatment of patients with relapsed or refractory multiple myeloma will be terminated early and SAR'514/IPH6401 will now be refocused to pursue development in autoimmune indications.
IPH62 and other target
- IPH62 is a NK-cell engager program targeting B7-H3 under development from Innate's ANKET platform. Following a research collaboration period and upon candidate selection, Sanofi will be responsible for all development, manufacturing and commercialization.
- Sanofi still retains the option of one additional ANKET target under the terms of the 2022 research collaboration and license agreement.
Antibody Drug Conjugates:
IPH4502 (Nectin-4 ADC, proprietary):
IPH4502 is Innate's novel and differentiated topoisomerase I inhibitor ADC targeting Nectin-4.
- The first patient was dosed in a Phase 1 study in January 2025. The Phase 1 will assess the safety, tolerability, and preliminary efficacy of IPH4502 in advanced solid tumors known to express Nectin-4, including but not limited to urothelial carcinoma, non-small cell lung, breast, ovarian, gastric, esophageal, and colorectal cancers. The study plans to enroll approximately 105 patients. A Trial in Progress Poster will be shared at the upcoming American Society of Clinical Oncology (ASCO) Annual Meeting in June 2025.
- New preclinical data were presented at the American Association for Cancer Research (AACR) Annual Meeting 2025. IPH4502 demonstrated superior preclinical anti-tumor activity compared to enfortumab vedotin (EV) in urothelial carcinoma models with low or heterogeneous Nectin-4 expression, as well as in models resistant to EV. Beyond UC, IPH4502 also exhibited anti-tumor activity in preclinical models of triple-negative breast cancer, head and neck squamous cell carcinoma, and esophageal cancer, suggesting broader potential clinical applicability.
Lacutamab (anti-KIR3DL2 antibody, proprietary):
Cutaneous T Cell Lymphoma
TELLOMAK is a global, open-label, multi-cohort Phase 2 clinical trial evaluating lacutamab in patients with Sézary syndrome and mycosis fungoides.
- In February 2025, the FDA granted Breakthrough Therapy Designation to lacutamab for relapsed or refractory Sézary syndrome based on TELLOMAK Phase 2 results demonstrating efficacy and a favorable safety profile in patients with advanced Sézary syndrome, heavily pretreated, post-mogamulizumab. Breakthrough Therapy Designation is intended to accelerate the development and regulatory review in the U.S. of drugs that are intended to treat a serious condition. Partnering discussions are underway. Phase 3 preparation is advancing with the FDA and EMA.
- Long-term follow-up for Sezary syndrome and mycosis fungoides will be presented at the ASCO Annual Meeting in June 2025.
Peripheral T Cell lymphoma (PTCL)
The Phase 2 KILT (anti-KIR in T Cell Lymphoma) trial, an investigator-sponsored, randomized controlled trial led by the Lymphoma Study Association (LYSA) to evaluate lacutamab in combination with chemotherapy GEMOX (gemcitabine and oxaliplatin) versus GEMOX alone in patients with KIR3DL2-expressing relapsed/refractory PTCL is ongoing and continues to recruit patients.
Monalizumab (anti-NKG2A antibody), partnered with AstraZeneca:
- The Phase 3 PACIFIC-9 trial run by AstraZeneca evaluating durvalumab (anti-PD-L1) in combination with monalizumab or AstraZeneca's oleclumab (anti-CD73) in patients with unresectable, Stage III non-small cell lung cancer (NSCLC) who have not progressed following definitive platinum-based concurrent chemoradiation therapy (CRT) is ongoing.
- AstraZeneca will present an update on the outcomes of the Phase 2 study NeoCOAST-2 at the upcoming ASCO Annual Meeting in June 2025. NeoCOAST-2 is evaluating neoadjuvant durvalumab plus chemotherapy and novel anticancer agents and adjuvant durvalumab with or without novel agents in resectable non-small-cell lung cancer.
IPH5201 (anti-CD39), partnered with AstraZeneca:
- The MATISSE Phase 2 clinical trial conducted by Innate in neoadjuvant lung cancer for IPH5201, an anti-CD39 blocking monoclonal antibody developed in collaboration with AstraZeneca, is ongoing and recruitment is on track.
IPH5301 (anti-CD73):
- The investigator-sponsored CHANCES Phase 1 trial of IPH5301 with Institut Paoli-Calmettes is ongoing.
Corporate Update
- As of March 31, 2025, the balance available under our April 2023 sales agreement under the At-The-Market program remains at $75 million.
Post period event
- As announced on April 23, 2025, as part of the discussions with regard to the review of their 2016 Agreement, Sanofi and Innate agreed to a potential investment by Sanofi of up to €15 million in new shares of Innate. Sanofi then subscribed to 8,345,387 new ordinary shares of Innate, at a price of €1.7974 per share, representing a total capital increase of €14,999,998.59 (€417,269.35 in nominal amount and €14,582,729.24 of issue premium), representing 9.05% of Innate's total outstanding shares as of the time of such capital increase.
- Innate Pharma will propose to its Annual General Meeting taking place on May 22, 2025, to move from an executive board/supervisory board corporate governance structure to a board of directors structure with a Chief Executive Officer. This transformation is part of the Company's strategic plan to simplify and align its governance with international standards.
Financial Results
Cash, cash equivalents and financial assets of the Company amounted to €72.5 million as of March 31, 2025. At the same date, financial liabilities amounted to €29.2 million. Cash, cash equivalents and financial assets as of March 31, 2025 do not include the €15.0 million payment received from Sanofi.
Revenue for the three month period ending March 31, 2025, amounted to €1.2 million (€6.6 million for the same period in 2024). Revenue from collaboration and licensing agreements mainly resulted from the partial or entire recognition of the proceeds received pursuant to the agreements with AstraZeneca and Sanofi.
About Innate Pharma
Innate Pharma S.A. is a global, clinical-stage biotechnology company developing immunotherapies for cancer patients. Its innovative approach aims to harness the innate immune system through three therapeutic approaches: multi-specific NK Cell Engagers via its ANKET (Antibody-based NK cell Engager Therapeutics) proprietary platform and Antibody Drug Conjugates (ADC) and monoclonal antibodies (mAbs).
Innate's portfolio includes several ANKET drug candidates to address multiple tumor types as well as IPH4502, a differentiated ADC in development in solid tumors. In addition, anti-KIR3DL2 mAb lacutamab is developed in advanced form of cutaneous T cell lymphomas and peripheral T cell lymphomas, and anti-NKG2A mAb monalizumab is developed with AstraZeneca in non-small cell lung cancer.
Innate Pharma is a trusted partner to biopharmaceutical companies such as Sanofi and AstraZeneca, as well as leading research institutions, to accelerate innovation, research and development for the benefit of patients.
Headquartered in Marseille, France with a US office in Rockville, MD, Innate Pharma is listed on Euronext Paris and Nasdaq in the US.
Learn more about Innate Pharma at www.innate-pharma.com. Follow us on LinkedIn and X.
Information about Innate Pharma shares
ISIN code Ticker code LEI | FR0010331421 Euronext: IPH Nasdaq: IPHA 9695002Y8420ZB8HJE29 |
Disclaimer on forward-looking information and risk factors
This press release contains certain forward-looking statements, including those within the meaning of applicable securities laws, including the Private Securities Litigation Reform Act of 1995. The use of certain words, including "anticipate," "believe," "can," "could," "estimate," "expect," "may," "might," "potential," "underway," "intend," "should," "will," or the negative of these and similar expressions, is intended to identify forward-looking statements. Although the Company believes its expectations are based on reasonable assumptions, these forward-looking statements are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those anticipated. These risks and uncertainties include, among other things, the uncertainties inherent in research and development, including related to safety, progression of and results from its ongoing and planned clinical trials and preclinical studies, review and approvals by regulatory authorities of its product candidates, the Company's reliance on third parties to manufacture its product candidates, the Company's commercialization efforts and the Company's continued ability to raise capital to fund its development. For an additional discussion of risks and uncertainties, which could cause the Company's actual results, financial condition, performance or achievements to differ from those contained in the forward-looking statements, please refer to the Risk Factors ("Facteurs de Risque") section of the Universal Registration Document filed with the French Financial Markets Authority ("AMF"), which is available on the AMF website http://www.amf-france.org or on Innate Pharma's website, and public filings and reports filed with the U.S. Securities and Exchange Commission ("SEC"), including the Company's Annual Report on Form 20-F for the year ended December 31, 2024, and subsequent filings and reports filed with the AMF or SEC, or otherwise made public by the Company. References to the Company's website and the AMF website are included for information only and the content contained therein, or that can be accessed through them, are not incorporated by reference into, and do not constitute a part of, this press release.
In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by the Company or any other person that the Company will achieve its objectives and plans in any specified time frame or at all. The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
This press release and the information contained herein do not constitute an offer to sell or a solicitation of an offer to buy or subscribe to shares in Innate Pharma in any country.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250512043208/en/
Contacts:
For additional information, please contact:
Investors
Innate Pharma
Henry Wheeler
Tel.: +33 (0)4 84 90 32 88
Henry.wheeler@innate-pharma.fr
Media Relations
NewCap
Arthur Rouillé
Tel.: +33 (0)1 44 71 00 15
innate@newcap.eu