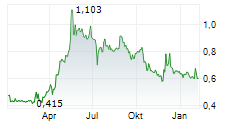
In Q125, Cereno made tangible progress across both its clinical programmes. The period opened with new data for lead asset CS1 indicating disease-modifying signals, and was capped by FDA endorsement of the Phase IIb plan (May 2025). The next step will be an IND application for Phase IIb and we anticipate data from the extended access programme (EAP, expected by mid-2025) to bolster the regulatory dossier. With Phase I now complete for second asset CS014 and a top-line readout in June, we expect a catalyst-rich period ahead for Cereno. The SEK77m gross cash and anticipated SEK50m from the November 2024 bridge loan should provide a runway into 2026. We expect Cereno to begin partnering talks in advance of trial initiation in H126. We raise our probability of success for CS1 to 45% (from 40%) based on recent developments. We upgrade our valuation to SEK4.5bn (SEK16.0/share) from SEK4.0bn (SEK14.2/share) previously.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research