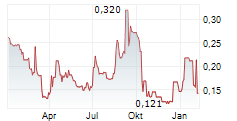
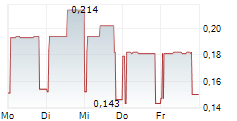
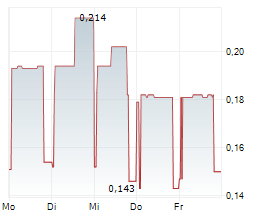
Sareum Holdings has reported the initiation of toxicology studies required ahead of the Phase II studies for its lead TYK2/JAK1 asset, SDC-1801. We view this as a key milestone in SDC-1801's development plan, with results likely to bolster any upcoming discussions with potential licensing partners. The studies will evaluate SDC-1801's general toxicology and potential drug-drug interactions, with completion expected in Q4 CY25. With a cash runway into 2026, the company is well funded to reach this milestone. This news follows Sareum's recent decision to test its TYK2/JAK1 programme in central nervous system (CNS) indications, following supportive preclinical data. TYK2/JAK1 mediate the activity of neuroinflammatory cytokines, such as IL-6, IL-12, IL-23, IFN-a/ß, and are emerging as potential targets in CNS, a space that has seen a resurgence in activity and investor interest.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research