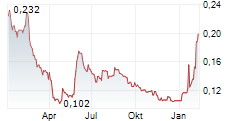
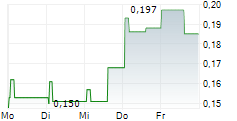
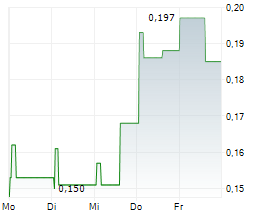
Creo Medical has received 510(k) FDA clearance for its latest endoscopic electrosurgical device, SpydrBlade Flex, strengthening its strategic foothold in the high-value US market. SpydrBlade Flex is a next-generation electrosurgical tool designed for flexible endoscopes, enabling both laparoscopic dissection and coagulation functionality. Following its European launch in Q324, commercial roll-out began in March 2025 in the UK, with the initial focus on gastrointestinal (GI) indications. This regulatory clearance comes shortly after the American Medical Association (AMA) approved two new reimbursement codes for upper and lower GI endoscopic submucosal dissection (ESD) procedures. We expect these codes to facilitate broader procedural adoption of Creo's electrosurgical devices, including SpydrBlade Flex, providing top-line traction.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research