CINCINNATI, Ohio, June 11, 2025 (GLOBE NEWSWIRE) -- Onconetix, Inc. (NASDAQ: ONCO) ("Onconetix" or the "Company"), a commercial-stage biotechnology company focused on the research, development, and commercialization of innovative solutions for men's health and oncology, today announced that the Company's stockholders have approved a proposal to effect a reverse split, which was voted on at the Company's 2025 special meeting of stockholders (the "Special Meeting") held on May 30, 2025, and that its Board of Directors (the "Board of Directors" or "Board") approved a 1-for-85 reverse stock split of its outstanding shares of common stock, to be effective as of 12:01 a.m. Eastern Time on June 13, 2025.
Results of the Special Meeting
At the Special Meeting, Onconetix's stockholders approved the following proposals:
- an amendment to the Onconetix, Inc. Amended and Restated Certificate of Incorporation to effect a reverse stock split of all of the outstanding shares of the Company's common stock, par value $0.00001 per share, at a ratio in the range of 1-for-10 to 1-for-150, at any time prior to the one-year anniversary date of the Special Meeting, with such ratio to be determined by the Board without further approval or authorization of the stockholders; and
- the adjournment of the Special Meeting, if necessary or appropriate, to solicit additional proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock Split Proposal. (the "Adjournment Proposal").
Final voting results from the Special Meeting were reported in a Current Report on Form 8-K filed with the Securities and Exchange Commission (the "SEC") on June 5, 2025.
Reverse Stock Split
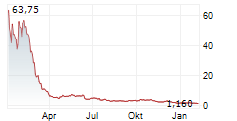
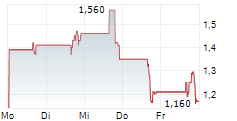
In conjunction with stockholder approval of the reverse stock split, the Company's Board of Directors determined to fix a split ratio of 1-for-85 shares. The Company's common stock will begin trading on a reverse stock split-adjusted basis at the opening of the market on June 13, 2025. Following the reverse stock split, the Company's common stock will continue to trade on The Nasdaq Capital Market under the symbol "ONCO" under the new CUSIP number 68237Q203. The reverse stock split is intended to enable the Company to regain compliance with the minimum bid price requirement of $1.00 per share of common stock for continued listing on The Nasdaq Capital Market.
At the effective time of the reverse split, every 85 issued and outstanding shares of the Company's common stock will be converted automatically into one share of the Company's common stock without any change in the par value per share. No fractional shares will be issued in connection with the reverse stock split, and fractional shares resulting from the reverse stock split will be canceled with the holders thereof receiving cash compensation. The amount of compensation will be determined by multiplying the fractional share by the closing price per share of the Company's common stock on The Nasdaq Capital Market at the close of business on the trading day prior to the effective date of the reserve stock split, or June 12, 2025. The reverse split will have no effect on the number of authorized shares of the Company's common stock, and the ownership percentage of each stockholder will remain unchanged other than as a result of fractional shares. The reverse stock split will additionally apply to the Company's common stock issuable upon exercise or conversion of the Company's equity awards, convertible preferred stock and warrants, as well as the applicable exercise price.
The reverse stock split will reduce the number of outstanding shares of the Company's common stock from approximately 44.4 million to approximately 521,863.
About Onconetix, Inc.
Onconetix, Inc. is a commercial-stage biotechnology company focused on the research, development, and commercialization of innovative solutions for men's health and oncology. The Company owns Proclarix, an in vitro diagnostic test for prostate cancer originally developed by Proteomedix and approved for sale in the European Union under the In Vitro Diagnostic Regulation. The Company also owns ENTADFI, an FDA-approved, once-daily pill that combines finasteride and tadalafil for the treatment of benign prostatic hyperplasia, a disorder of the prostate. For more information, visit www.onconetix.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as "anticipate," "believe," "forecast," "estimate," "expect," and "intend," among others. These forward-looking statements (including, without limitation, statements regarding the timing and effectiveness of the anticipated reverse split and compliance with applicable Nasdaq continued listing requirements) are based on Onconetix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, market and other conditions; whether the Company will be able to regain and maintain compliance with Nasdaq's applicable listing criteria and the effect of a delisting from Nasdaq on the market for the Company's securities; whether a definitive agreement for the proposed transaction with Ocuvex Therapeutics, Inc. ("Ocuvex") and any related financing will be entered into; whether such transactions, or any other contemplated transaction, may be completed with different terms, in an untimely manner, or not at all; whether the Company will be able to realize the benefits of a proposed transaction with Ocuvex; Onconetix's ability to integrate the assets and commercial operations contemplated to be acquired from Ocuvex into the Company's business; risks related to Onconetix's ability to commercialize or monetize Proclarix and integrate the assets and commercial operations; risks related to the Company's present need for capital to commercially launch Proclarix and have adequate working capital; risks related to Onconetix's ability to attract, hire and retain skilled personnel necessary to commercialize and operate the Company's commercial products; the failure to obtain and maintain the necessary regulatory approvals to market and commercialize Onconetix's products; risks related to the Company's ability to obtain and maintain intellectual property protection for its current products; and the Company's reliance on third parties, including manufacturers and logistics companies. As with any commercial-stage pharmaceutical product or any product candidate under clinical development, there are significant risks in the development, regulatory approval and commercialization of biotechnology products. Onconetix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in Onconetix's Annual Report on Form 10-K, filed with the SEC on June 2, 2025 and periodic reports filed with the SEC on or after the date thereof. All of Onconetix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
For more information:
Onconetix, Inc.
201 E. Fifth Street, Suite 1900
Cincinnati, OH 45202
Phone: (513) 620-4101
Investor Contact Information:
Onconetix Investor Relations
Email: investors@onconetix.com