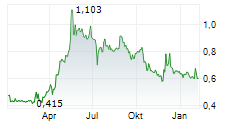
Cereno Scientific has announced encouraging four-month follow-up data from its Expanded Access Program (EAP) for CS1, a first-in-class HDAC inhibitor being developed to treat pulmonary arterial hypertension (PAH). While details have not been disclosed, management has noted that the data, collected from a 10-patient cohort who continued CS1 treatment following the successful Phase IIa trial, align closely with earlier results, reinforcing the drug's safety and tolerability, as well as early efficacy signals. Top-line results from the full 12-month follow-up are expected in Q126, and we believe these, along with data from the Fluidda sub-study, will help build understanding of long-term usage of CS1 as the company prepares for the Phase IIb study, to commence in H126. Our estimates remain unchanged following this announcement, as we await the full 12-month results in Q126.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research