Suspension of CARMAT shares trading starting June 30, 2025, before stock market opening
Regulatory News:
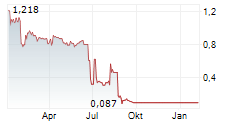
CARMAT (FR0010907956, ALCAR), designer and developer of the world's most advanced total artificial heart, aiming to provide a therapeutic alternative for people suffering from advanced biventricular heart failure (the "Company" or "CARMAT"), today announces filing for insolvency1 and requesting to be placed in receivership2 to the Versailles Economic Affairs Court3, as well as the suspension of CARMAT shares trading, starting June 30, 2025, before stock market opening.
Insolvency filing and request to be placed in receivership
On June 20, 2025, CARMAT announced in a press release being at risk of insolvency as early as the end of June 2025 unless managing before then, to secure additional cash of at least €3.5 million.
Despite its continued efforts, the Company has not managed at this stage, to secure neither additional cash nor new financing.
Given this, the Company will today file for insolvency and request to be placed in receivership to the Versailles Economic Affairs Court ("the Court").
The Court will rule on this request, following a hearing expected in the coming days.
As a reminder, according to its current business plan and assuming "business as usual" situation, the Company estimates its funding requirements over the next 12 months at approximately €35 million, including approximately €20 million by the end of December 2025.
Suspension of CARMAT shares trading (ISIN code: FR0010907956, Ticker: ALCAR)
Pending the Court's decision, CARMAT has asked Euronext to suspend the trading of its shares starting on June 30, 2025, before the stock market opens.
The Company anticipates this suspension to be lifted once the Court's decision has been rendered and communicated to the market.
Next steps
Pending the Court's decision, CARMATS's operations carry-on and the Company continues to actively explore all options to ensure the continuation of its business activities.
The Company believes that the opening of a receivership procedure would be the most appropriate framework to facilitate this continuation.
More generally, whatever the Court's decision, the Company will endeavor to provide continuous support to patients who currently benefit from its Aeson® artificial heart.
Press releases will be issued regularly as the Company's situation evolves and the proceedings progress.
About CARMAT
CARMAT is a French MedTech that designs, manufactures and markets the Aeson artificial heart. The Company's ambition is to make Aeson the first alternative to a heart transplant, and thus provide a therapeutic solution to people suffering from end-stage biventricular heart failure, who are facing a well-known shortfall in available human grafts. The world's first physiological artificial heart that is highly hemocompatible, pulsatile and self-regulated, Aeson could save, every year, the lives of thousands of patients waiting for a heart transplant. The device offers patients quality of life and mobility thanks to its ergonomic and portable external power supply system that is continuously connected to the implanted prosthesis. Aeson is commercially available as a bridge to transplant in the European Union and other countries that recognize CE marking. Aeson is also currently being assessed within the framework of an Early Feasibility Study (EFS) in the United States. Founded in 2008, CARMAT is based in the Paris region, with its head offices located in Vélizy-Villacoublay and its production site in Bois-d'Arcy. The Company can rely on the talent and expertise of a multidisciplinary team of circa 200 highly specialized people. CARMAT is listed on the Euronext Growth market in Paris (Ticker: ALCAR ISIN code: FR0010907956).
For more information, please go to www.carmatsa.com and follow us on LinkedIn.
Name: CARMAT
ISIN code: FR0010907956
Ticker: ALCAR
Disclaimer
This press release and the information it contains do not constitute an offer to sell or subscribe, nor a solicitation of an offer to buy or subscribe, for CARMAT shares in any country.
This press release may contain forward-looking statements regarding the Company's objectives and outlook. These forward-looking statements are based on the current estimates and anticipations of the Company's management and are subject to risk factors and uncertainties, including those described in its Universal Registration Document filed with the French Financial Markets Authority (Autorité des marchés financiers) (the "AMF") under number D.25-0345 (the "2024 Universal Registration Document"), available free of charge on the websites of CARMAT (www.carmatsa.com/en/) and the AMF (www.amf-france.org).
Readers' attention is particularly drawn to the fact that the Company's current cash runway extends only until the end of June 2025, and that CARMAT is therefore facing a very high risk of default, including in the very short term. The Company is also exposed to other risks and uncertainties, such as its ability to implement its strategy, the pace of development of its production and sales, the progress and results of ongoing or planned clinical trials, technological developments, the competitive landscape, regulatory changes, industrial risks, and all risks related to the management of the Company's growth. Forward-looking statements mentioned in this press release may not be achieved due to these factors or other unknown risks and uncertainties, or risks that the Company does not currently consider to be material or specific.
Aeson® is an active implantable medical device commercially available in the European Union and other countries recognising the CE mark. The Aeson® total artificial heart is intended to replace the ventricles of the native heart and is indicated as a bridge to transplant in patients with end-stage biventricular heart failure (Intermacs classes 1-4) who cannot benefit from maximal medical therapy or a left ventricular assist device (LVAD) and who are likely to benefit from a heart transplant within 180 days of implantation. The decision to implant and the surgical procedure must be carried out by healthcare professionals trained by the manufacturer. The documentation (clinician's manual, patient's manual and alarm booklet) must be read carefully to learn about the characteristics of Aeson® and the information required for patient selection and proper use (contraindications, precautions, side effects) of Aeson®. In the United States, Aeson® is currently only available as part of a feasibility clinical trial approved by the Food Drug Administration (FDA).
1 | Déclaration de cessation des paiements | |
2 | Redressement judiciaire | |
3 | Tribunal des Affaires Economiques de Versailles |
View source version on businesswire.com: https://www.businesswire.com/news/home/20250629186076/en/
Contacts:
CARMAT
Stéphane Piat
Chief Executive Officer
Pascale d'Arbonneau
Deputy Chief Executive Officer Chief Financial Officer
Tel.: +33 1 39 45 64 50
contact@carmatsas.com
NewCap
Press Relations
Nicolas Merigeau
Arthur Rouillé
Tel.: +33 1 44 71 94 98
carmat@newcap.eu
NewCap
Financial Communication
& Investor Relations
Dusan Oresansky
Jérémy Digel
Tel.: +33 1 44 71 94 92
carmat@newcap.eu