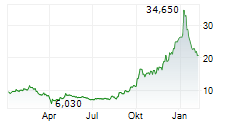
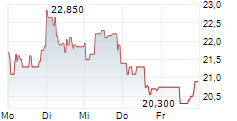
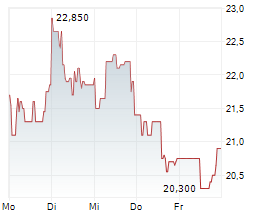
Newron Pharmaceuticals is making significant headway in progressing its drug candidate, evenamide, to become an effective treatment option for patients suffering with treatment-resistant schizophrenia (TRS). The latest update from the company confirmed that the registrational ENIGMA-TRS Phase III programme (expected n=1,000) will consist of two separate studies (versus a single trial as originally planned). The first of these commenced within H125 (according to management), and it is anticipated to report top-line results from Q426. We update our estimates to reflect the second international Phase III study and adjust our model to factor in self-commercialisation in the US. Our valuation adjusts to CHF392.4m or CHF19.7/share, from CHF385.6m or CHF19.3/share previously.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research