London, United Kingdom--(Newsfile Corp. - July 9, 2025) - Edison issues report on Newron Pharmaceuticals (SIX: NWRN).
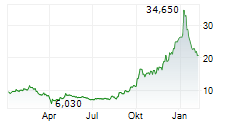
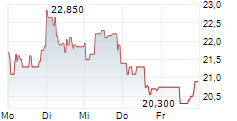
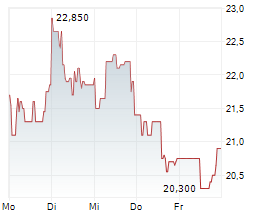
Newron Pharmaceuticals is making significant headway in progressing its drug candidate, evenamide, to become an effective treatment option for patients suffering with treatment-resistant schizophrenia (TRS). The latest update from the company confirmed that the registrational ENIGMA-TRS Phase III programme (expected n=1,000) will consist of two separate studies (versus a single trial as originally planned). The first of these commenced within H125 (according to management), and it is anticipated to report top-line results from Q426. We update our estimates to reflect the second international Phase III study and adjust our model to factor in self-commercialisation in the US. Our valuation adjusts to CHF392.4m or CHF19.7/share, from CHF385.6m or CHF19.3/share previously.
Click here to read the full report.
All reports published by Edison are available to download free of charge from its website
www.edisongroup.com
Edison is authorised and regulated by the Financial Conduct Authority.
Edison is not an adviser or broker-dealer and does not provide investment advice. Edison's reports are not solicitations to buy or sell any securities.
For more information, please contact Edison:
enquiries@edisongroup.com
Connect with Edison on:
LinkedIn www.linkedin.com/company/edison-group-/
X www.x.com/edison_inv_res
YouTube www.youtube.com/edisonitv

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/258250
SOURCE: Edison Group