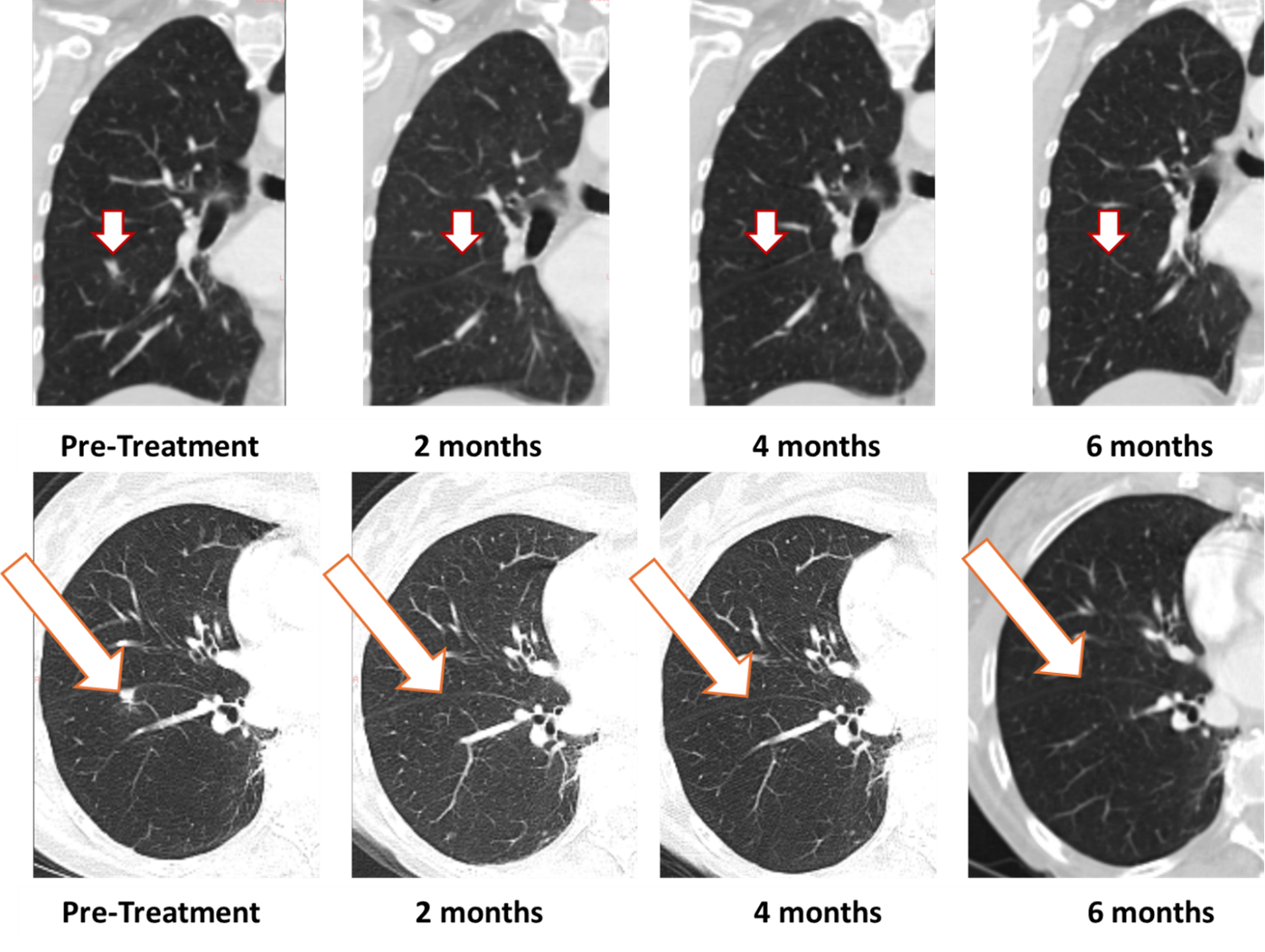
- [IMAGES BELOW] Complete resolution maintained at 6 months in first patient treated with BriaCell's Bria-OTS in Phase 1/2a study
- No treatment limited toxicities observed
- Patient remains on study with stable disease elsewhere
PHILADELPHIA and VANCOUVER, British Columbia, July 09, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW, BCTXZ) (TSX: BCT) ("BriaCell" or the "Company"), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, today announced the sustained complete resolution of lung metastasis in a patient with hormone receptor-positive (HR+), HER2-negative, metastatic breast cancer (MBC) treated with Bria-OTS, the Company's personalized off the shelf immunotherapy.
BriaCell's first Bria-OTS study patient, a 78-year-old woman with advanced disease and multiple prior treatment failures, achieved 100% resolution of a lung metastasis following four doses of BriaCell's Bria-OTS monotherapy. The complete response was first observed at two months (previously reported) and confirmed at four (previously reported) and now six months. The patient has been dosed with 12 cycles of Bria-OTS to date.
Figure 1: Treatment with Bria-OTS monotherapy resulted in 100% resolution of tumor in the right lung of the metastatic breast cancer (MBC) patient following 2 months of therapy and confirmed at 4, and 6 months of therapy1 (axial and coronal views)
"These results represent an exciting clinical milestone in the Bria-OTS program," stated Neal S. Chawla MD, Director at the Sarcoma Oncology Center, Santa Monica, CA, and Principal Investigator for the Bria-OTS study. "We are seeing strong single agent activity in a very challenging population and are eager to explore this approach across more patient subtypes and tumors."
"We are highly encouraged by this remarkable and durable clinical response, especially at the lowest dose level," added Dr. William V. Williams, BriaCell's President and CEO. "This data underscores the therapeutic potential of our Bria-OTS platform, and we look forward to further evaluating it in combination with a checkpoint inhibitor to improve outcomes in patients with advanced breast cancer."
About Bria-OTS
Bria-OTS is a next generation, off-the-shelf personalized immunotherapy based on BriaCell's lead candidate Bria-IMT currently being evaluated in a Phase 1/2a study (ClinicalTrials.gov identifier: NCT06471673) in patients with metastatic recurrent breast cancer. The trial includes both monotherapy dose escalation and check point inhibition combination dose expansion cohorts. The Company recently progressed into the dose expansion phase.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release include statements regarding: BriaCell continuing the Phase 1/2a Bria-OTS study and reproducing similar results in patients with MBC and other cancers; the use of the Bria-OTS platform as monotherapy; and Bria-OTS's validation as a personalized immunotherapy approach. Forward-looking statements may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading "Risks and Uncertainties" in the Company's most recent Management's Discussion and Analysis, under the heading "Risk Factors" in the Company's most recent Annual Information Form, and under "Risks and Uncertainties" in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Investor Relations Contact:
investors@briacell.com
1 Note that the other white dots in the lungs are blood vessels.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/086d3bd1-2e71-468f-9d93-f7ebafff2797.