Dual siRNA Therapeutic Confirms Its Robust In Vivo Results, Strongly De-Risking the Clinical Path and Underscoring CCT-217's Potential as a Transformative, Patient-Friendly Therapy for Obesity and Metabolic Disorders
BOSTON, MASSACHUSETTS / ACCESS Newswire / July 10, 2025 / Canary Cure Therapeutics (Canary), a pioneering biopharmaceutical company, today announced groundbreaking human cell data for its lead therapeutic candidate, CCT-217. The findings confirm a unique mechanism that promotes rapid, selective fat loss while preserving and building muscle. This strong human and preclinical evidence de-risks the clinical path for a transformative, twice-yearly treatment for obesity, with Phase 1 trials targeted for 2026.
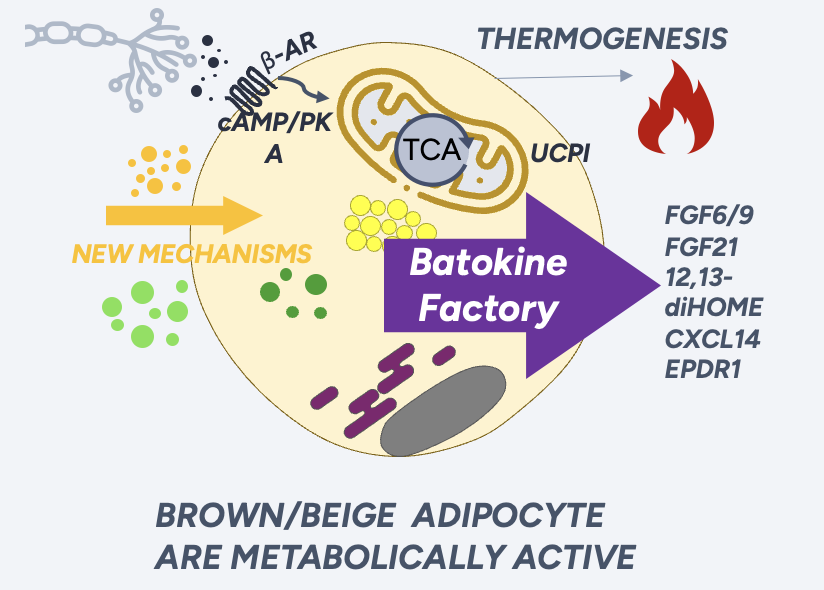
The global obesity market is projected to exceed $200 billion by 2031, yet current treatments primarily rely on appetite suppression, often leading to significant loss of healthy muscle. CCT-217 represents a paradigm shift by targeting the underlying metabolic causes of obesity.
"While weight loss can be achieved by decreasing calorie intake, we believe increasing the body's ability to burn fat - especially harmful visceral fat - is a fundamentally healthier approach," said Raj Reddy, CEO of Canary. "Our goal is to improve body composition and overall metabolic function."
Developed from Canary's proprietary dual siRNA platform, CCT-217 is engineered to increase energy expenditure by converting energy-storing white fat into metabolically active, energy-burning beige fat.
Highlights From Recent Data:
Human Cell Data Confirms Mechanism: Recent studies on differentiated human white fat cells treated with CCT-217 showed a rapid and significant increase in key thermogenic gene markers within just 72 hours. This powerful result in human cells validates the therapy's core mechanism and potential efficacy, providing a clear path toward clinical trials.
Dramatic, Fat-Selective Weight Loss in Preclinical Models: In diet-induced obese (DIO) animal models, CCT-217 demonstrated remarkable results in only 20 days:
26% reduction in total body weight
60% reduction in harmful visceral fat
25% increase in lean muscle mass
Comprehensive Metabolic Health Improvements: Beyond weight loss, CCT-217 treatment led to a complete reversal of liver steatosis (fatty liver), a 20% reduction in cholesterol, and enhanced insulin sensitivity, underscoring its potential to address a wide range of obesity-related co-morbidities.
CCT-217 offers patient-friendly, twice-yearly subcutaneous injections, using a proven Lipid Nanoparticle (LNP) platform to target siRNA delivery to adipose tissue - an endocrine organ central to the development of metabolic diseases - while minimizing off-target effects. Canary's second asset, CCT-007, targets genes over-expressed in post-menopausal women for healthy weight loss with fewer side effects.
"The strong correlation between our new human cell data and the dramatic in vivo animal model outcomes provides critical de-risking evidence," said Dr. Reddy. "We are now focused on advancing CCT-217 into Phase 1 clinical trials in 2026 to bring this next-generation therapy to patients. We are fortunate to have exceptional advisors overseeing the clinical development including Dr. Michael Lincoff, Principal Investigator of the groundbreaking SELECT Trial."
Canary is currently raising a Series A round of $25 million to advance CCT-217 into Phase 1 trials, expected to start in 2026.
About Canary Cure Therapeutics
Canary Cure Therapeutics is a late-stage preclinical biopharmaceutical company pioneering next-generation RNA therapeutics for obesity and metabolic disease. Its proprietary dual-siRNA platform is engineered to induce class-leading thermogenesis, offering a differentiated, muscle-sparing approach to weight loss and metabolic health.
Contact Information
Anna Wang
Chief Strategy Officer
anna@canarycure.com
+41 79 204 2875
SOURCE: Canary Cure Therapeutics
View the original press release on ACCESS Newswire:
https://www.accessnewswire.com/newsroom/en/biotechnology/canary-cure-therapeutics-unveils-breakthrough-human-cell-data-for-next-generation-obe-1047486
