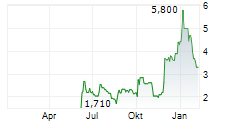
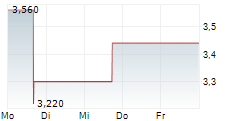
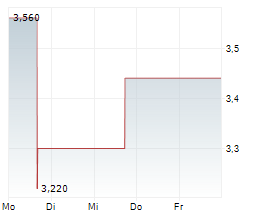
Vancouver, British Columbia--(Newsfile Corp. - July 17, 2025) - NervGen Pharma Corp. (TSXV: NGEN) (OTCQB: NGENF) (the "Company"), a clinical-stage biotechnology company developing innovative therapies for spinal cord injury (SCI) and other nervous system disorders, today announced a leadership transition as the Company enters the next phase of development for its first and potential best-in-class candidate, NVG-291.
President and Chief Executive Officer Mike Kelly has stepped down as a director and officer of the Company and Dr. Adam Rogers, Chair of the Board and representative of NervGen's largest shareholder, has been appointed Interim CEO.
"We are deeply grateful to Mike for his exceptional leadership during a pivotal time for NervGen," said Dr. Adam Rogers, Chairman and Interim CEO of NervGen. "Under Mike's guidance, the Company advanced NVG-291 through landmark proof-of-concept topline results from the chronic cohort of the CONNECT SCI study. Mike's leadership established the organizational framework needed for future growth and set a clear strategic direction to position NervGen one step closer to addressing its mission of transforming the lives of individuals living with spinal cord injury."
"The chronic cohort of the CONNECT SCI study represents the strongest signal of efficacy observed to date in spinal cord injury," Dr. Rogers added. "We are entering the most exciting and important phase in NervGen's history and are committed to proactively engaging with regulators and the SCI community to advance NVG-291 toward its full potential."
Regarding his departure, Mr. Kelly commented, "It's been an honor to lead NervGen during a pivotal time of clinical and operational growth. I'm proud of the team we've built and the opportunity to successfully advance NVG-291 through an unprecedented proof-of-concept efficacy trial in chronic spinal cord injury. With positive data in hand, NervGen is well-positioned for future growth. I remain a strong advocate of the Company and its mission to redefine the standard of care in spinal cord injury."
The leadership transition comes as NervGen continues to analyze data from the chronic cohort of the CONNECT SCI study in preparation for sharing additional insights and engaging in regulatory discussions.
About NVG-291
NervGen holds exclusive worldwide rights to NVG-291, a first- and potential best-in-class therapeutic peptide targeting nervous system repair. NVG-291's technology is licensed from Case Western Reserve University and is based on academic studies that demonstrated the preclinical efficacy of NVG-291-R, the rodent variant of NVG-291, in animal models of spinal cord injury. These studies implicated multiple potential molecular and cellular mechanisms by which NVG-291-R promotes neurorepair and functional improvement in both central and peripheral nervous system injury models. The implicated mechanisms include the promotion of neuronal sprouting, or plasticity, remyelination, and promotion of a non-inflammatory phenotype in the microglial cells. NervGen has received Fast Track designation from the FDA and Orphan Designation from the EMA for NVG-291 in individuals with spinal cord injury.
About NervGen
NervGen (TSXV: NGEN) (OTCQB: NGENF) is a clinical-stage biotechnology company dedicated to developing innovative therapies to promote nervous system repair in settings of neurotrauma and neurologic disease. The Company is testing the clinical efficacy of its lead candidate, NVG-291, in the Phase 1b/2a CONNECT SCI Study clinical trial in spinal cord injury. Topline data from the chronic cohort (1-10 years post-injury) of this trial showed that NVG-291 met its primary endpoint and demonstrated strong trends in functional assessments.
Continued analysis of data from the chronic cohort is ongoing. Enrollment in the subacute cohort (20-90 days post-injury) of the trial continues, and more information about participation in the subacute study is available at www.connectscistudy.com. In addition, the company has initiated preclinical testing of concept evaluation of its pipeline candidate, NVG-300, in models of ischemic stroke and spinal cord injury. For more information about NervGen, visit www.nervgen.com and follow NervGen on X and LinkedIn for the latest news on the company.
Contacts
Huitt Tracey, Investor Relations
htracey@nervgen.com
604.537.2094
Bill Adams, Chief Financial Officer
info@nervgen.com
778.731.1711
Christy Curran
Sam Brown Healthcare Communications
christycurran@sambrown.com
615.414.8668
646.942.5604
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Cautionary Note Regarding Forward-Looking Statements
This news release may contain "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian and United States securities legislation (collectively, "forward-looking statements"). Such forward-looking statements herein include but are not limited to, the Company's current and future plans, expectations and intentions, results, levels of activity, performance, goals or achievements, or any other future events or developments constitute forward-looking statements, and the words "may", "will", "would", "should", "could", "expect", "plan", "intend", "trend", "indication", "anticipate", "believe", "estimate", "predict", "likely" or "potential", or the negative or other variations of these words or other comparable words or phrases, are intended to identify forward-looking statements. Forward-looking statements include, without limitation, statements relating to: the Company's potential best-in-class candidate, NVG-291; the future growth of the Company; the Company's mission of transforming the lives of individuals living with spinal cord injury; the objectives, planned clinical endpoints, timing, expected rate of enrollment, and study design of our Phase 1b/2a clinical trial of NVG-291 in individuals with spinal cord injury; the future development plans and benefits of NVG-291; the development plans and prospective target indications for NVG-300; and the creation of neuroreparative therapeutics to promote nervous system repair in settings of neurotrauma and neurologic disease. Forward-looking statements are based on estimates and assumptions made by the company in light of management's experience and perception of historical trends, current conditions and expected future developments, as well as other factors that we believe are appropriate and reasonable in the circumstances. In making forward-looking statements, we have relied on various assumptions, including, but not limited to: our ability to obtain future funding on favorable terms or at all; the accuracy of our financial projections; obtaining positive results in our clinical and other trials; our ability to obtain necessary regulatory approvals; our ability to arrange for the manufacturing of our product candidates and technologies; and general business, market and economic conditions. Many factors could cause our actual results, level of activity, performance or achievements or future events or developments to differ materially from those expressed or implied by the forward-looking statements, including without limitation, a lack of revenue, insufficient funding, reliance upon key personnel, the uncertainty of the clinical development process, competition, and other factors set forth in the "Risk Factors" section of the company's most recently filed prospectus supplement, short form base shelf prospectus, annual information form, financial statements and management discussion and analysis all of which can be found on NervGen's profile on SEDAR+ at www.sedarplus.ca. All clinical development plans are subject to additional funding. Readers should not place undue reliance on forward-looking statements made in this news release. Furthermore, unless otherwise stated, the forward-looking statements contained in this news release are made as of the date of this news release, and we have no intention and undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. The forward-looking statements contained in this news release are expressly qualified by this cautionary statement.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/259101
SOURCE: NervGen Pharma Corp.