With in-vivo CAR-T therapies gaining global momentum, a new whitepaper from Novotech, a leading global full-service clinical Contract Research Organization (CRO) and scientific advisory company, offers insight into this next frontier of immunotherapy. In-Vivo CAR Therapies Global Research and Development Landscape (2025) report explores the accelerating innovation in in-vivo CAR-T (Chimeric Antigen Receptor) technologies and their potential to reshape treatment across oncology, autoimmune diseases, and other complex indications.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250717625155/en/
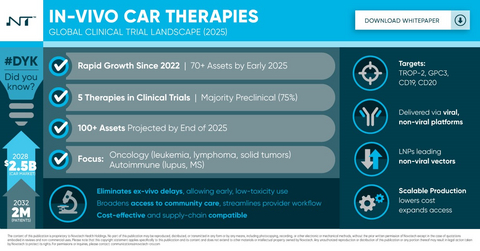
In Vivo Car T Infographic from Novotech
Traditional ex-vivo CAR-T therapies require extracting a patient's T cells, engineering them in specialized labs, and reinfusing them, a process that is time-intensive, expensive, and logistically complex. These barriers have significantly restricted patient access.
In contrast, in-vivo CAR-T therapies aim to overcome these challenges by engineering T cells directly within the patient's body using delivery technologies such as viral vectors, lipid nanoparticles (LNPs), and advanced mRNA platforms. The result: faster, more scalable, and potentially off-the-shelf treatments that can expand access, reduce costs, and streamline the patient experience.
In-vivo CAR-T platforms are now being explored not only for hematologic malignancies, but also for solid tumors and autoimmune diseases-areas where traditional CAR-T approaches have faced limitations. Emerging delivery innovations and new combination strategies are driving this expansion.
As a result, in-vivo CAR-T therapies are positioned to reshape clinical practice, expanding their applicability across oncology, autoimmune diseases, and beyond.
The report provides key insights for sponsors, investors, and researchers into one of the most transformative areas of next-generation cell therapy. It includes:
- The technological evolution of CAR platforms and delivery systems.
- The expanding global pipeline of in-vivo CAR assets and clinical programs.
- Innovations in lipid nanoparticle (LNP) and viral vector delivery strategies.
- Commercial and regulatory considerations shaping future adoption.
- Opportunities for in-vivo CAR approaches beyond oncology, including autoimmune diseases and fibrosis.
Novotech has an established track record of supporting in-vivo CAR-T programs globally, including conducting the world's first in-vivo CAR clinical trial, highlighting its leadership and deep expertise in advanced therapeutics, including gene and cell therapies. The company has also conducted more than 100 ATMP studies, spanning gene therapies, gene-modified cell therapies, and mRNA products. Novotech is also actively partnering with biotech sponsors advancing in-vivo CAR pipelines.
Download the full whitepaper to explore the key trends and opportunities driving the future of in-vivo CAR-T therapy.
About Novotech Novotech-CRO.com
Novotech is a globally recognized full-service clinical research organization (CRO) and scientific advisory company trusted by biotech and small- to mid-sized pharmaceutical companies to guide drug development at every phase.
With a global footprint that includes 30+ offices across the Asia-Pacific region, North America, and Europe and partnerships with 5,000+ trial sites, Novotech provides clients an accelerated path to bring life-changing therapies to market by providing access to key clinical trial destinations and diverse patient populations.
Through its client-centric service model, Novotech seamlessly integrates people, processes, and technologies to deliver customized solutions that accelerate the path to market for life-changing therapies. By adopting a true partnership approach, Novotech shares a steadfast commitment to client success, empowering innovation, and advancing healthcare worldwide.
Recipient of numerous industry accolades, including the Frost Sullivan CRO Company of the Year award for 19 consecutive years, Novotech is recognized for its excellence in clinical trial execution and innovation. Its deep therapeutic and regulatory expertise, combined with local market insights, ensures streamlined clinical trials, optimized data analytics, and accelerated patient recruitment strategies.
Together with clients, Novotech transforms scientific advancements into therapies that improve global health outcomes, embodying a mission of driving innovation and delivering impactful results.
For more information or to speak to an expert team member visit www.Novotech-CRO.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250717625155/en/
Contacts:
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135
