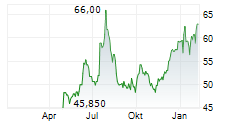
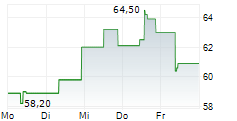
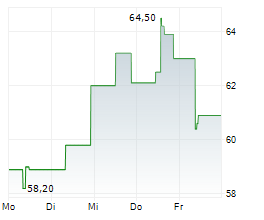
Basilea Pharmaceutica has commenced the second Phase III study for fosmanogepix, a key step forward for its novel broad-spectrum antifungal candidate. The trial (FORWARD-IM) will evaluate the efficacy and safety of fosmanogepix in invasive, multi-drug-resistant mould infections against the standard of care (SoC) across two cohorts (n=220). In contrast to the ongoing placebo-controlled FAST-IC trial in invasive yeast infections, FORWARD-IM is an open-label study, raising the possibility of interim data readouts, ahead of top-line results expected in 2028. Fosmanogepix, a first-in-class agent with a novel mechanism of action, has demonstrated promising safety and efficacy across three completed Phase II studies and remains one of the broadest-spectrum antifungals in development. We maintain our valuation and estimates for Basilea following this milestone.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research