LIBERATE-1, the first-in-human application of Vivani's NanoPortalTM implant technology, showed a positive safety and tolerability profile, along with encouraging performance data for NPM-115 that met the study's primary objectives
New NPM-139 (semaglutide implant) preclinical feasibility data showed approximately 20% weight loss maintained longer than 6 months with a single implant, continuing to support the potential for annual dosing
Based on these data, Vivani prioritizes the advancement of NPM-139, with clinical development expected to begin in 2026, pending regulatory clearance
ALAMEDA, Calif., Aug. 05, 2025 (GLOBE NEWSWIRE) -- Vivani Medical, Inc. (Nasdaq: VANI) ("Vivani" or the "Company"), a clinical-stage, biopharmaceutical company developing miniature, ultra long-acting drug implants, today reported results from the LIBERATE-1 clinical study, the Phase 1 study of the exenatide GLP-1 implant NPM-115 representing the first-in-human test of the Company's proprietary NanoPortal implant technology.
The Company also reported new feasibility data for NPM-139 (semaglutide implant) from an ongoing preclinical study, supporting prioritization of the semaglutide implant in the Company's pipeline and clinical development strategy. Semaglutide is the active ingredient in blockbuster drug products Ozempic®, Wegovy®, and Rybelsus®.
Vivani Chief Executive Officer Adam Mendelsohn, Ph.D. stated, "We are very pleased to report that LIBERATE-1, our Phase 1 study in obese and overweight subjects and the first clinical application of NanoPortal technology, achieved its primary objectives. The results support the general safety and tolerability profile of the device and continued development with higher dose configurations, which we expect to produce clinically relevant weight management effects."
"In addition to the LIBERATE-1 results, we are very excited to report new preclinical feasibility data with our semaglutide implant candidate NPM-139, under development for chronic weight management which have shown approximately 20% weight loss with a single administration for over 6 months and continue to show potential for annual dosing. When we couple the successful completion of the LIBERATE-1 study with the new feasibility data for our semaglutide implant, it is an easy decision to focus our resources and prioritize efforts to accelerate NPM-139 into clinical-stage development."
The decision to prioritize NPM-139 is supported by several factors. These include the Company's belief that the development timelines for the NPM-115 and NPM-139 programs are comparable, the increased confidence in NPM-139 due to the fact that semaglutide products have already established compelling weight loss data in humans, and the strong commercial performance of semaglutide-based products, including Ozempic, Wegovy, and Rybelsus, which have generated over $29B in sales in 2024 and are expected to have continued growth into the foreseeable future.
LIBERATE-1 Study Results
The LIBERATE-1 Phase 1 study successfully met its primary objectives, which were to evaluate the NPM-115 implant's safety and tolerability profile and to characterize the pharmacokinetic (PK) profile of the implant over a 9-week duration. Throughout the study, the implant was generally well tolerated, and drug release from the implant without any clinically meaningful burst was supported by PK analysis and by the absence of gastrointestinal adverse events in subjects with the implant. No serious adverse events were observed in the study. The release profile observed from the implants over 9 weeks provides encouragement regarding the potential for this technology to provide durable delivery over the 6-month duration that has already been established in preclinical studies of both NPM-115 and NPM-139.
This study paves the way for future clinical development of the implant technology not just for exenatide (NPM-115 and NPM-119) but also for semaglutide (NPM-139) and any other application of NanoPortal technology that Vivani may pursue in the future.
Semaglutide Implant Preclinical Feasibility Data
In an ongoing preclinical study, substantial progress in the development of a miniature, ultra long-acting, semaglutide implant, NPM-139 has been established by showing weight loss from a single administration for over 231 days.
NPM-139 (Semaglutide Implant)
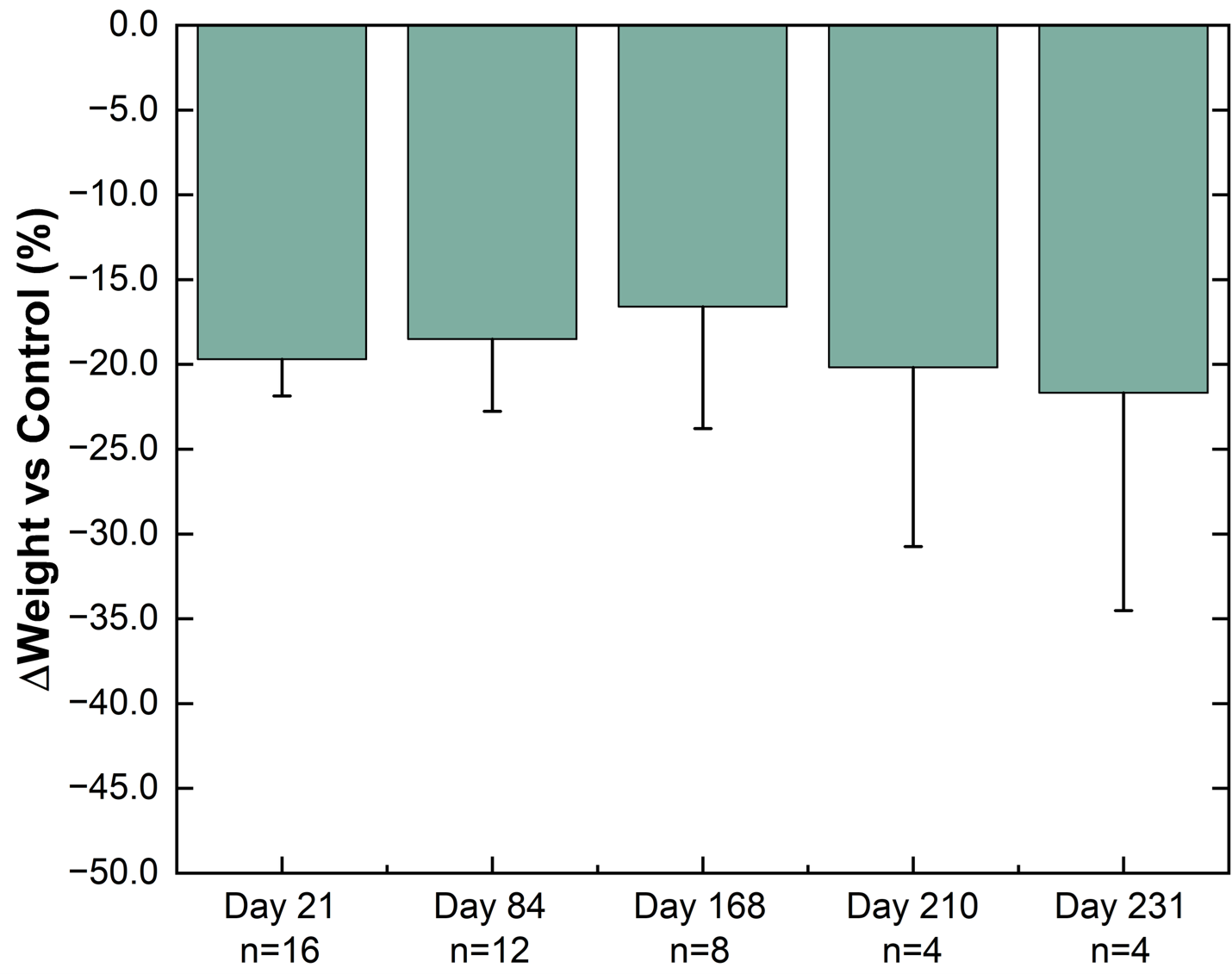
Weight difference versus control group in healthy Sprague-Dawley rats. The percentage weight change from baseline for NPM-139 (semaglutide implant) corrected to control (sham implant). Implants from 4 animals were removed on each of Day 21, Day 84, and Day 168 for characterization. Values are mean ± standard error.
While the emerging preclinical data on the Company's semaglutide implant currently supports the initial target profile of bi-annual dosing, the Company continues to anticipate that a semaglutide implant candidate may be able to support annual dosing in the future. Vivani's near-term efforts are focused on completion of PK optimization activities and preparation of data to enable the submission of an Investigational New Drug application for NPM-139.
About Vivani Medical, Inc.
Leveraging its proprietary NanoPortal platform, Vivani develops biopharmaceutical implants designed to deliver drug molecules steadily over extended periods of time with the goal of guaranteeing adherence and improving patient tolerance to their medication. Vivani's priority product candidate, NPM-139, is a miniature, six-month, subdermal, GLP-1 (semaglutide) implant under development for chronic weight management in obese or overweight subjects. NPM-139 has the added potential for once-yearly dosing. Vivani's emerging pipeline also includes NPM-115 (exenatide implant) for chronic weight management in obese and overweight individuals, and NPM-119, an exenatide implant program for the treatment of type-2 diabetes. The Company is also considering another semaglutide implant for the treatment of type 2 diabetes. These NanoPortal implants are designed to provide patients with the opportunity to realize the full potential benefit of their medication by avoiding the numerous challenges associated with the daily or weekly administration of orals and injectables, including tolerability issues and loss of efficacy. Medication non-adherence occurs when patients do not take their medication as prescribed. This affects an alarming number of patients, approximately 50%, including those taking daily pills.
Forward-Looking Statements
This press release contains certain "forward-looking statements" within the meaning of the "safe harbor" provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "target," "believe," "expect," "will," "may," "anticipate," "estimate," "would," "positioned," "future," and other similar expressions that in this press release, including statements regarding Vivani's business, products in development, including the therapeutic potential thereof, the planned development therefor, the completion of the LIBERATE-1 Phase 1 study and reporting of study results, Vivani's emerging development plans for NPM-139, NPM-115, NPM-119 or Vivani's plans with respect its technology, strategy, cash position and financial runway. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on Vivani's current beliefs, expectations, and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of Vivani's control. Actual results and outcomes may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and outcomes to differ materially from those indicated in the forward-looking statements include, among others, risks related to the development and commercialization of Vivani's products, including NPM-139, NPM-115, and NPM-119; delays and changes in the development of Vivani's products, including as a result of applicable laws, regulations and guidelines, potential delays in submitting and receiving regulatory clearance or approval to conduct Vivani's development activities, including Vivani's ability to commence clinical development of NPM-139; risks related to the initiation, enrollment and conduct of Vivani's planned clinical studies and the results therefrom; or Vivani's history of losses and Vivani's ability to access additional capital or otherwise fund Vivani's business. There may be additional risks that the Company considers immaterial, or which are unknown. A further list and description of risks and uncertainties can be found in the Company's most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission on March 31, 2025, as updated by the Company's subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement made by Vivani in this press release is based only on information currently available to the Company and speaks only as of the date on which it is made. The Company undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of added information, future developments or otherwise, except as required by law.
Company Contact:
Donald Dwyer
Chief Business Officer
info@vivani.com
(415) 506-8462
Investor Relations Contact:
Jami Taylor
Investor Relations Advisor
investors@vivani.com
(415) 506-8462
Media Contact:
Mark Corbae
ICR Healthcare
Mark.Corbae@ICRHealthcare.com
(203) 682-8288
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8a049bc2-9622-4156-b325-dc73a1f1b9d1.