- Reported Total Revenues of $19.5 million for the second quarter of 2025, exceeding the high end of the Company's guidance range of between $17 million and $19 million
- SERB Pharmaceuticals to acquire Y-mAbs in $412.0 million transaction; transaction expected to close by the fourth quarter of 2025, subject to completion of tender offer and other customary conditions
- As of June 30, 2025, cash and cash equivalents were $62.3 million
- In light of the pending transaction, Y-mAbs will not be holding a webcast and conference call to discuss its second quarter 2025 results
PRINCETON, N.J., Aug. 08, 2025 (GLOBE NEWSWIRE) -- Y-mAbs Therapeutics, Inc. (the "Company" or "Y-mAbs") (Nasdaq: YMAB), a commercial-stage biopharmaceutical company focused on the development and commercialization of novel radioimmunotherapy and antibody-based therapeutic products for the treatment of cancer, today reported financial results for the second quarter ended June 30, 2025.
Recent Transaction Announcement with SERB
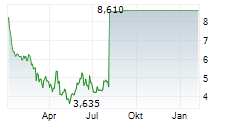
- On August 5, 2025, Y-mAbs announced it has entered into a definitive agreement with affiliated entities of SERB Pharmaceuticals ("SERB"), under which SERB, a global specialty pharmaceutical company, agreed to acquire Y-mAbs in a transaction at an equity value of $412.0 million. The transaction was unanimously approved by the Y-mAbs Board of Directors. Under the terms of the merger agreement, SERB is obligated to commence a tender offer by August 19, 2025 to purchase all outstanding shares of Y-mAbs for $8.60 per share in cash, representing a 105% premium to Y-mAbs' closing share price on August 4, 2025, the last full trading day prior to the transaction announcement.
- The transaction is expected to close by the fourth quarter of 2025, assuming a majority of outstanding Y-mAbs shares are tendered into, and not withdrawn from, the tender offer, and subject to the satisfaction of customary conditions, including the receipt of a majority of Y-mAbs shares in the tender offer and expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act.
Financial Results
Revenues
Total revenues for the quarter ended June 30, 2025 were $19.5 million, which was a 14% decrease from the $22.8 million of total revenues for the quarter ended June 30, 2024, driven by a $2.9 million decrease in Ex-U.S. DANYELZA® net product revenues and a $0.9 million decrease in U.S. DANYELZA net product revenues, partially offset by a $0.5 million increase related to license revenue recognized in the three months ended June 30, 2025. Total DANYELZA net product revenues for the quarter ended June 30, 2025 were $19.0 million, which was a 17% decrease over $22.8 million total DANYELZA net product revenues for the quarter ended June 30, 2024.
Total revenues for the six months ended June 30, 2025 were $40.4 million, which was a 5% decrease from the $42.7 million of total revenues for the six months ended June 30, 2024, driven by a $6.1 million decrease in U.S DANYELZA net product revenues, partially offset by a $3.8 million increase in Ex-U.S. DANYELZA net product revenues. The total revenues in the six months ended June 30, 2025 and 2024 include $0.5 million license revenue, respectively.
The Company's U.S. DANYELZA net product revenues for the quarter ended June 30, 2025 were $14.3 million, representing a decrease of 6% from the same period in 2024. The decline in the U.S. DANYELZA net product revenues was driven by declining patient volume due to enrollments in clinical studies and competition in the three months ended June 30, 2025.
The Company's Ex-U.S. DANYELZA net product revenues for the quarter ended June 30, 2025 were $4.7 million, representing a decrease of $2.9 million from the same period in 2024. Ex-U.S. DANYELZA net product revenues include $2.1 million from Western Europe and $3.4 million from Eastern Asia for the three months ended June 30, 2024, which was a result of stocking orders from Western Europe and Eastern Asia in 2024. The Company did not have any stocking orders from Western Europe or Eastern Asia in the three months ended June 30, 2025. The decrease in Ex-U.S. DANYELZA net product revenues was partially offset by a $2.0 million increase in DANYELZA net product revenues in Western Asia, where a named patient program launched in Turkey in late 2024.
During the three and six months ended June 30, 2025, the Company recognized $0.5 million license revenue, which was earned in prior periods, in connection with sales-based milestone achievements by our partner in Israel. There was no license revenue in the three months ended June 30, 2024. During the six months ended June 30, 2024, the Company had license revenues of $0.5 million, which included license revenue from the Latin America distribution partner, Adium, related to price approval for DANYELZA in Brazil from the Brazilian Medicines Market Regulation Chamber.
Cost of Goods Sold
Cost of goods sold was $2.7 million and $3.0 million for the three months ended June 30, 2025 and 2024, respectively. The decrease in cost of goods sold in the three months ended June 30, 2025 compared to the same period in 2024 was primarily driven by decreased sales.
Cost of goods sold was $5.7 million and $5.1 million for the six months ended June 30, 2025 and 2024, respectively. The increase in cost of goods sold in the six months ended June 30, 2025 compared to the same period in 2024 was primarily driven by increased cost of production.
Gross Profit
Gross profit stayed consistent at $16.8 million and $19.8 million for the three months ended June 30, 2025 and 2024, respectively. Gross margins were relatively flat at 86% and 87% for the three months ended June 30, 2025 and 2024, respectively.
Gross profit was $34.8 million and $37.6 million for the six months ended June 30, 2025 and 2024, respectively. Gross margins were 86% and 88% for the six months ended June 30, 2025 and 2024, respectively. The gross margin decreased primarily due to increased cost of production and lower product sales to Western Europe, where product sales generally have higher gross margin.
Operating Costs and Expenses
Research and Development
Research and development expenses were $11.1 million and $12.3 million for the three months ended June 30, 2025 and 2024, respectively. The $1.2 million decrease in research and development expenses was primarily driven by a decrease of $0.9 million in personnel and stock-based compensation costs which was primarily related to our business realignment announced in January 2025.
Research and development expenses were $22.5 million and $25.6 million for the six months ended June 30, 2025 and 2024, respectively. The $3.1 million decrease in research and development expenses was primarily driven by a $1.8 million decrease in personnel and stock-based compensation costs, which was primarily related to our business realignment announced in January 2025, and a total $0.4 million decrease in clinical trials and outsourced research and supplies due to the timing of completion in our GD2-SADA program and investment in our ongoing SADA PRIT programs.
Selling, General, and Administrative
Selling, general, and administrative expenses were $11.3 million and $17.2 million for the three months ended June 30, 2025 and 2024, respectively. The $5.9 million decrease in selling, general and administrative expenses was primarily attributable to a net impact of $3.8 million related to litigation settlements during the three months ended June 30, 2024 and a $1.7 million decrease in legal expenses.
Selling, general, and administrative expenses were $24.4 million and $28.7 million for the six months ended June 30, 2025 and 2024, respectively. The $4.3 million decrease in selling, general, and administrative expenses was primarily attributable to a net impact of $3.8 million related to litigation settlements in the six months ended June 30, 2024, as noted above.
Interest and Other Income
Interest and other income were $2.3 million and $0.6 million for the three months ended June 30, 2025 and 2024, respectively. Interest and other income increased by $1.7 million primarily due to $2.0 million of foreign currency transaction gains in the three months ended June 30, 2025, partially offset by a $0.3 million decrease in interest earned on cash and cash equivalents.
Interest and other income were $3.7 million and $1.1 million for the six months ended June 30, 2025 and 2024, respectively. Interest and other income increased by $2.6 million primarily due to $3.3 million of foreign currency transaction gains, partially offset by a $0.7 million decrease in interest earned on cash and cash equivalents.
Net Loss
Y-mAbs reported a net loss for the quarter ended June 30, 2025, of $3.2 million, or ($0.07) per basic and diluted share, compared to a net loss of $9.2 million, or ($0.21) per basic and diluted share, for the quarter ended June 30, 2024. The decrease in net loss for the quarter ended June 30, 2025 was primarily driven by the above noted $3.8 million in litigation settlements in the quarter end June 30, 2024 and the favorable impact of $2.0 million of foreign currency transaction gains in the quarter ended June 30, 2025.
Cash and Cash Equivalents
As of June 30, 2025, Y-mAbs had approximately $62.3 million in cash and cash equivalents. The Company continues its efforts to be capital efficient in its operations.
In light of the pending transaction, Y-mAbs will not be holding a webcast and conference call to discuss its second quarter 2025 results.
About Y-mAbs
Y-mAbs is a commercial-stage biopharmaceutical company focused on the development and commercialization of novel, radioimmunotherapy and antibody-based therapeutic cancer products. The Company's broad and advanced commercial product pipeline includes the anti-GD2 therapy DANYELZA® (naxitamab-gqgk), the first FDA-approved treatment for patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow after a partial response, minor response, or stable disease to prior therapy. The Company's technologies include its investigational Self-Assembly DisAssembly ("SADA") Pretargeted Radioimmunotherapy Platform ("PRIT") and bispecific antibodies generated using the Y-BiClone platform.
Forward-Looking Statements
This press release contains forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Y-mAbs and SERB, including statements relating to the ability to complete and the timing of completion of the transactions contemplated by the Agreement and Plan of Merger dated as of August 4, 2025 by and among Y-mAbs, SERB, and the other parties thereto (the "Merger Agreement"), including the anticipated occurrence, manner and timing of the proposed tender offer, the parties' ability to satisfy the conditions to the consummation of the tender offer and the other conditions to the consummation of the subsequent merger set forth in the Merger Agreement, the possibility of any termination of the Merger Agreement, and the prospective benefits of the proposed transaction; and other statements that are not historical facts. The forward-looking statements contained in this release are based on current expectations and assumptions that are subject to risks and uncertainties which may cause actual results to differ materially from the forward-looking statements. These statements may contain words such as "may," "will," "would," "could," "expect," "anticipate," "intend," "plan," "believe," "estimate," "project," "seek," "should," "strategy," "future," "opportunity," "potential" or other similar words and expressions indicating future results. Risks that may cause these forward-looking statements to be inaccurate include, without limitation: uncertainties as to the timing of the tender offer; uncertainties as to how many of Y-mAbs' stockholders will tender their stock in the offer; the possibility that competing offers or acquisition proposals will be made; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay, or refuse to grant approval for the consummation of the transaction (or only grant approval subject to adverse conditions or limitations); the difficulty of predicting the timing or outcome of regulatory approvals or actions, if any; the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement; the possibility that the transaction does not close; risks related to the parties' ability to realize the anticipated benefits of the proposed transaction, including the possibility that the expected benefits from the proposed acquisition will not be realized or will not be realized within the expected time period and that Y-mAbs and SERB will not be integrated successfully or that such integration may be more difficult, time-consuming or costly than expected; the effects of the transaction on relationships with employees, customers, suppliers, other business partners or governmental entities; negative effects of this announcement or the consummation of the proposed transaction on the market price of Y-mAbs' common stock and/or Y-mAbs' operating results; significant transaction costs; unknown or inestimable liabilities; the risk of litigation and/or regulatory actions related to the proposed transaction; SERB's ability to fund the proposed transaction; obtaining and maintaining adequate coverage and reimbursement for products; the time-consuming and uncertain regulatory approval process; the costly and time-consuming pharmaceutical product development process and the uncertainty of clinical success, including risks related to failure or delays in successfully initiating or completing clinical trials and assessing patients; global economic, financial, and healthcare system disruptions and the current and potential future negative impacts to the parties' business operations and financial results; the sufficiency of the parties' cash flows and capital resources; the parties' ability to achieve targeted or expected future financial performance and results and the uncertainty of future tax, accounting and other provisions and estimates; and other risks and uncertainties affecting Y-mAbs and SERB, including those described from time to time under the caption "Risk Factors" and elsewhere in Y-mAbs' filings and reports with the U.S. Securities and Exchange Commission (the "SEC"), including Y-mAbs' Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2025 and June 30, 2025, as well as the Tender Offer Statement on Schedule TO and related tender offer documents to be filed by SERB and its acquisition subsidiary, and the Solicitation/Recommendation Statement on Schedule 14D-9 to be filed by Y-mAbs. Any forward-looking statements are made based on the current beliefs and judgments of Y-mAbs' and SERB's management, and the reader is cautioned not to rely on any forward-looking statements made by Y-mAbs or SERB. Except as required by law, Y-mAbs and SERB do not undertake any obligation to update (publicly or otherwise) any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
DANYELZA® and Y-mAbs® are registered trademarks of Y-mAbs Therapeutics, Inc.
| Y-MABS THERAPEUTICS, INC. Consolidated Balance Sheets (unaudited) (In thousands, except share and per share data) | ||||||||
| June 30, | December 31, | |||||||
| 2025 | 2024 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 62,293 | $ | 67,234 | ||||
| Accounts receivable, net | 15,740 | 19,688 | ||||||
| Inventories | 9,719 | 7,214 | ||||||
| Other current assets | 4,035 | 4,373 | ||||||
| Total current assets | 91,787 | 98,509 | ||||||
| Property and equipment, net | 269 | 42 | ||||||
| Operating lease right-of-use assets | 3,109 | 817 | ||||||
| Intangible assets, net | 2,177 | 2,276 | ||||||
| Inventories, long-term | 19,223 | 17,772 | ||||||
| Other assets | 646 | 488 | ||||||
| TOTAL ASSETS | $ | 117,211 | $ | 119,904 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| LIABILITIES | ||||||||
| Accounts payable | $ | 7,571 | $ | 6,662 | ||||
| Accrued liabilities | 14,888 | 16,406 | ||||||
| Operating lease liabilities, current portion | 486 | 630 | ||||||
| Total current liabilities | 22,945 | 23,698 | ||||||
| Accrued milestones | 3,200 | 3,200 | ||||||
| Operating lease liabilities, long-term portion | 2,638 | 190 | ||||||
| Other liabilities | 935 | 812 | ||||||
| TOTAL LIABILITIES | 29,718 | 27,900 | ||||||
| STOCKHOLDERS' EQUITY | ||||||||
| Preferred stock, $0.0001 par value, 5,500,000 shares authorized and none issued at June 30, 2025 and December 31, 2024 | - | - | ||||||
| Common stock, $0.0001 par value, 100,000,000 shares authorized at June 30, 2025 and December 31, 2024; 45,438,420 and 44,988,313 shares issued and outstanding at June 30, 2025 and December 31, 2024, respectively | 5 | 4 | ||||||
| Additional paid-in capital | 583,671 | 576,872 | ||||||
| Accumulated other comprehensive income | (612 | ) | 2,264 | |||||
| Accumulated deficit | (495,571 | ) | (487,136 | ) | ||||
| TOTAL STOCKHOLDERS' EQUITY | 87,493 | 92,004 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ | 117,211 | $ | 119,904 | ||||
| Y-MABS THERAPEUTICS, INC. Consolidated Statements of Net Loss and Comprehensive Loss (unaudited) (In thousands, except share and per share data) | ||||||||||||||||
| Three months ended June 30, | Six months ended June 30, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| REVENUES | ||||||||||||||||
| Net product revenue | $ | 19,025 | $ | 22,798 | $ | 39,929 | $ | 42,229 | ||||||||
| License revenue | 500 | - | 500 | 500 | ||||||||||||
| Total revenues | 19,525 | 22,798 | 40,429 | 42,729 | ||||||||||||
| COST OF GOODS SOLD | 2,662 | 3,014 | 5,663 | 5,111 | ||||||||||||
| GROSS PROFIT | 16,863 | 19,784 | 34,766 | 37,618 | ||||||||||||
| OPERATING COSTS AND EXPENSES | ||||||||||||||||
| License royalties | 50 | - | 50 | 50 | ||||||||||||
| Research and development | 11,104 | 12,341 | 22,463 | 25,608 | ||||||||||||
| Selling, general, and administrative | 11,313 | 17,232 | 24,400 | 28,657 | ||||||||||||
| Total operating costs and expenses | 22,467 | 29,573 | 46,913 | 54,315 | ||||||||||||
| Loss from operations | (5,604 | ) | (9,789 | ) | (12,147 | ) | (16,697 | ) | ||||||||
| OTHER INCOME, NET | ||||||||||||||||
| Interest and other income | 2,372 | 640 | 3,723 | 1,079 | ||||||||||||
| LOSS BEFORE INCOME TAXES | (3,232 | ) | (9,149 | ) | (8,424 | ) | (15,618 | ) | ||||||||
| Provision for income taxes | 7 | 100 | 12 | 260 | ||||||||||||
| NET LOSS | $ | (3,239 | ) | $ | (9,249 | ) | $ | (8,436 | ) | $ | (15,878 | ) | ||||
| Other comprehensive income/(loss) | ||||||||||||||||
| Foreign currency translation | (2,013 | ) | 199 | (2,876 | ) | 598 | ||||||||||
| COMPREHENSIVE LOSS | $ | (5,252 | ) | $ | (9,050 | ) | $ | (11,312 | ) | $ | (15,280 | ) | ||||
| Net loss per share attributable to common stockholders, basic and diluted | $ | (0.07 | ) | $ | (0.21 | ) | $ | (0.19 | ) | $ | (0.36 | ) | ||||
| Weighted average common shares outstanding, basic and diluted | 45,318,028 | 44,022,356 | 45,212,065 | 43,900,639 | ||||||||||||
| Y-MABS THERAPEUTICS, INC. Consolidated Statements of Cash Flows (unaudited) (In thousands) | ||||||||
| Six months ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (8,436 | ) | $ | (15,878 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 124 | 312 | ||||||
| Stock-based compensation | 6,243 | 7,285 | ||||||
| Foreign currency transactions | (2,752 | ) | 724 | |||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable, net | 3,948 | 263 | ||||||
| Inventories | (2,394 | ) | (3,433 | ) | ||||
| Insurance recovery receivable related to legal settlement | - | (16,025 | ) | |||||
| Other current assets | 338 | 2,712 | ||||||
| Inventories, long-term | (1,451 | ) | (1,084 | ) | ||||
| Other assets | (158 | ) | 115 | |||||
| Accounts payable | 3,654 | 3,406 | ||||||
| Accrued liabilities and other | (4,376 | ) | (1,226 | ) | ||||
| Accrued legal settlement | - | 19,650 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (5,260 | ) | (3,179 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (127 | ) | - | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (127 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from exercised stock options | 446 | 2,346 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 446 | 2,346 | ||||||
| Effect of exchange rates on cash and cash equivalents | - | 2 | ||||||
| NET DECREASE IN CASH AND CASH EQUIVALENTS | (4,941 | ) | (831 | ) | ||||
| Cash and cash equivalents at the beginning of period | 67,234 | 78,637 | ||||||
| Cash and cash equivalents at the end of period | $ | 62,293 | $ | 77,806 | ||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH ACTIVITIES | ||||||||
| Right-of-use assets obtained in exchange for lease obligations | $ | 2,560 | $ | 320 | ||||
| Property and equipment purchase in accrued liabilities and other | $ | 118 | $ | - | ||||
| Y-MABS THERAPEUTICS, INC. Selected Financial Information by Reportable Segment (unaudited) (In thousands) | ||||||||||||||||||||||||
| Three Months Ended June 30, | ||||||||||||||||||||||||
| 2025 | 2024 | |||||||||||||||||||||||
| DANYELZA | RIT | Total | DANYELZA | RIT | Total | |||||||||||||||||||
| REVENUES | ||||||||||||||||||||||||
| Net product revenue | $ | 19,025 | $ | - | $ | 19,025 | $ | 22,798 | $ | - | $ | 22,798 | ||||||||||||
| License revenue | 500 | - | 500 | - | - | - | ||||||||||||||||||
| Total revenues | 19,525 | - | 19,525 | 22,798 | - | 22,798 | ||||||||||||||||||
| COST OF GOODS SOLD | 2,662 | - | 2,662 | 3,014 | - | 3,014 | ||||||||||||||||||
| OPERATING COSTS AND EXPENSES | ||||||||||||||||||||||||
| License royalties | 50 | - | 50 | - | - | - | ||||||||||||||||||
| Research and development | 5,444 | 5,239 | 10,683 | 5,185 | 5,835 | 11,020 | ||||||||||||||||||
| Selling, general, and administrative | 3,865 | 417 | 4,282 | 5,205 | - | 5,205 | ||||||||||||||||||
| Segment profit/(loss) from operations | $ | 7,504 | $ | (5,656 | ) | $ | 1,848 | $ | 9,394 | $ | (5,835 | ) | $ | 3,559 | ||||||||||
| Corporate and unallocated expenses - Research and development | 421 | 1,321 | ||||||||||||||||||||||
| Corporate and unallocated expenses - Selling, general, and administrative | 7,031 | 12,027 | ||||||||||||||||||||||
| Consolidated Loss from Operations | (5,604 | ) | (9,789 | ) | ||||||||||||||||||||
| OTHER INCOME, NET | ||||||||||||||||||||||||
| Corporate and unallocated expenses - Interest and other income | 2,372 | 640 | ||||||||||||||||||||||
| CONSOLIDATED LOSS BEFORE INCOME TAXES | $ | (3,232 | ) | $ | (9,149 | ) | ||||||||||||||||||
| Six Months Ended June 30, | ||||||||||||||||||||||||
| 2025 | 2024 | |||||||||||||||||||||||
| DANYELZA | RIT | Total | DANYELZA | RIT | Total | |||||||||||||||||||
| REVENUES | ||||||||||||||||||||||||
| Net product revenue | $ | 39,929 | $ | - | $ | 39,929 | $ | 42,229 | $ | - | $ | 42,229 | ||||||||||||
| License revenue | 500 | - | 500 | 500 | - | 500 | ||||||||||||||||||
| Total revenues | 40,429 | - | 40,429 | 42,729 | - | 42,729 | ||||||||||||||||||
| COST OF GOODS SOLD | 5,663 | - | 5,663 | 5,111 | - | 5,111 | ||||||||||||||||||
| OPERATING COSTS AND EXPENSES | ||||||||||||||||||||||||
| License royalties | 50 | - | 50 | 50 | - | 50 | ||||||||||||||||||
| Research and development | 10,370 | 10,935 | 21,305 | 10,594 | 11,876 | 22,470 | ||||||||||||||||||
| Selling, general, and administrative | 8,021 | 828 | 8,849 | 8,904 | - | 8,904 | ||||||||||||||||||
| Segment profit/(loss) from operations | $ | 16,325 | $ | (11,763 | ) | $ | 4,562 | $ | 18,070 | $ | (11,876 | ) | $ | 6,194 | ||||||||||
| Corporate and unallocated expenses - Research and development | 1,158 | 3,138 | ||||||||||||||||||||||
| Corporate and unallocated expenses - Selling, general, and administrative | 15,551 | 19,753 | ||||||||||||||||||||||
| Consolidated Loss from Operations | (12,147 | ) | (16,697 | ) | ||||||||||||||||||||
| OTHER INCOME, NET | ||||||||||||||||||||||||
| Corporate and unallocated expenses - Interest and other income | 3,723 | 1,079 | ||||||||||||||||||||||
| CONSOLIDATED LOSS BEFORE INCOME TAXES | $ | (8,424 | ) | $ | (15,618 | ) | ||||||||||||||||||