MELBOURNE, Australia and NEW YORK, Aug. 08, 2025 (GLOBE NEWSWIRE) -- Incannex Healthcare Inc. (Nasdaq: IXHL), a clinical-stage pharmaceutical company developing innovative combination therapies for prevalent medical conditions, today announced new patient-reported outcome findings from a subset of participants in its RePOSA Phase 2 trial of IHL-42X for the treatment of obstructive sleep apnoea (OSA). These new insights, collected through structured exit interviews, add to an already compelling data package highlighting the significant potential of IHL-42X to improve both clinical and patient-centric outcomes in individuals living with OSA.
The exit interviews were conducted in alignment with FDA guidance on Patient-Focused Drug Development (PFDD) and aimed to qualitatively assess patient experience, including the impact of IHL-42X on their sleep and quality of life. The interviews were completed prior to unblinding and included subjects from all three arms of the trial-placebo, low-dose IHL-42X, and high-dose IHL-42X.
Key findings from the exit interviews include:
- 57.6% of participants reported a perceived improvement in their OSA
- 89.5% of those reporting improvement described the change as meaningful to their lives
- Reported benefits included: improved sleep quality, feeling more refreshed in the morning, reduced daytime sleepiness and fatigue, fewer cognitive disturbances, and greater ease in completing daily responsibilities
Patients reported being motivated to participate in the trial due to a desire for improved health, better sleep, and access to alternatives to PAP (positive airway pressure) therapy. These patient insights add important context to the robust clinical data from the RePOSA Phase 2 trial.
Joel Latham, President and CEO of Incannex, commented:
"Our recent Phase 2 results for IHL-42X exceeded expectations. In some patients, we observed reductions in AHI of up to 83%, which is an extraordinary outcome and a powerful signal of the drug's potential."
"We believe the findings from the patient exit interviews underscore that these improvements are not just clinical-they are translating into meaningful, positive changes in patients' daily lives. This reinforces our belief that IHL-42X has the potential to significantly shift the treatment paradigm for OSA."
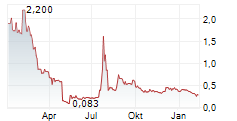
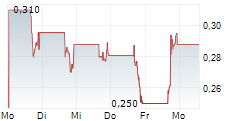
"However, despite the strength of our data and news flow, we do not believe our share price reflects the underlying value we've created. Like many small-cap companies, we are vulnerable to volatility-particularly from short-selling and trading that appears disconnected from company fundamentals. While we cannot comment on specific trades or potential manipulation, I want shareholders to know that we are actively monitoring the situation. We are also engaging with advisors and are committed to protecting shareholder value."
"From a financial standpoint, we remain well-capitalized to execute on our clinical programs. We are being disciplined with capital allocation and considering all strategic levers available to us to deliver long-term value-including those that reinforce confidence in our valuation and protect against dilution. I want to be very clear: our belief in the potential of IHL-42X has never been stronger."
Compelling Clinical Efficacy
The RePOSA Phase 2 trial demonstrated clear statistically and clinically significant improvements across multiple key endpoints for patients receiving IHL-42X compared to placebo, highlighting its potential to reduce OSA severity and enhance patient quality of life.
Apnoea-Hypopnoea Index (AHI): The low-dose and high-dose IHL-42X groups achieved a statistically significant reduction in percent change in AHI from baseline compared to placebo (p<0.05), the primary measure of OSA severity. Maximum reductions in AHI were observed at up to 83% for the high-dose group and up to 79% for the low-dose group.
=30% and =50% AHI Reduction Rates: 33.3% of patients in the low-dose group and 41.2% in the high-dose group achieved a greater than 30% reduction in AHI, while 13.9% (low-dose) and 14.7% (high-dose) experienced reductions exceeding 50%-demonstrating a strong therapeutic response in a substantial subset of the population.
Patient Global Impression of Change (PGI-C): Statistically significant improvements in sleep-related impairment and fatigue in the low-dose group (p<0.05), reflecting meaningful patient-perceived benefits.
Oxygen Desaturation Index (ODI): Statistically significant improvements in both low- and high-dose groups, indicating better oxygenation during sleep.
Patient-Reported Outcomes: Clinically significant improvements were observed in the Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10), PROMIS Sleep-Related Impairment 8a (PROMIS-SRI 8a), PROMIS Fatigue 7a, and Epworth Sleepiness Scale (ESS) in both dose groups, demonstrating enhanced sleep quality, reduced fatigue, and improved daily function.
Polysomnography (PSG) Metrics: IHL-42X also improved objective sleep parameters including Wake After Sleep Onset (WASO), which was reduced by 29.8% in the high-dose arm, and AHI During Supine Sleep, which decreased by 30.3% in the high-dose arm.
REM Sleep: IHL-42X did not reduce the proportion of time spent in REM sleep, as measured in the PSGs. This distinguishes IHL-42X from many drugs that are approved for other sleep indications, which are known to reduce the amount of time spent in REM sleep. REM is an important stage of sleep that contributes to memory consolidation, emotional regulation and brain health.
These results collectively demonstrate IHL-42X's ability to address both objective and subjective aspects of OSA, offering compelling clinical benefits to patients.
Outstanding Safety Profile
IHL-42X was well tolerated across both low- and high-dose cohorts. No serious adverse events were reported during the treatment period, and treatment-emergent adverse effects (TEAEs) were infrequent, with the majority being mild or moderate in severity. The Company believes this excellent safety profile supports IHL-42X's potential for broad patient use.
About Incannex Healthcare Inc.
Incannex is leading the way in developing combination medicines that target the underlying biological pathways associated with chronic conditions, including obstructive sleep apnea, rheumatoid arthritis and generalized anxiety disorder. The company is advancing three clinical-stage product candidates based on evidence-based innovation, and supported by streamlined operations. Incannex's lead clinical program, IHL-42X, is an oral fixed-dose combination of dronabinol and acetazolamide designed to target underlying mechanisms and act synergistically in the treatment of obstructive sleep apnea. In a Phase 2 development program, IHL-675A is an oral fixed-dose combination of cannabidiol and hydroxychloroquine sulfate designed to act synergistically to alleviate inflammatory conditions, such as rheumatoid arthritis. Approved for Phase 2 clinical development, PSX-001 is an oral synthetic psilocybin treatment for the treatment of generalized anxiety disorder. Incannex's programs target disorders that have limited, inadequate, or no approved pharmaceutical treatment options. For additional information on Incannex, please visit our website at www.incannex.com.
Forward Looking Statements
This press release contains "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements other than historical facts and relate to future events, future circumstances and Incannex's future performance. These statements are based on management's current assumptions, expectations, and beliefs. Examples of forward-looking statements in this press release include statements about, among other things: statements regarding potential future dilution; Incannex's opinions and estimates about the fundamentals and underlying value of its business relative to the trading price of its common stock; business strategy, future operations; Incannex's ability to execute on its objectives, prospects, commercial discussions or plans; evaluations and judgments regarding Incannex's research and development efforts and potential future commercialization, including any implications that the results (including qualitative patient-reported outcomes) of earlier clinical trials or interim or topline results will be representative or consistent with later clinical trials or their respective interim or final results; the potential benefits (including qualitative patient-reported outcomes) and safety of Incannex's drug candidates and the market opportunity for these candidates; and potential shareholder value. These forward-looking statements are subject to a number of risks and uncertainties, which may cause the forward-looking events and circumstances described in this press release to not occur, and actual results to differ materially and adversely from those described in or implied by the forward-looking statements. These risks and uncertainties include, among others: that current expense and cash resource estimates which may be ultimately be inaccurate and the Company may need to raise capital, including through the ATM, sooner than it presently anticipates; the continued availability of financing; Incannex's ability to raise capital to fund continuing operations and to maintain or potentially further improve its capital structure; Incannex's ability to maintain the listing of its shares of common stock on the Nasdaq Stock Market; the impact of any infringement actions or other litigation brought against Incannex; the success of Incannex's development efforts, including Incannex's ability to progress its drug candidates through clinical trials on the timelines expected and to obtain necessary regulatory approvals for commercialization of its product candidates; the effects of competition from other providers and products as currently existing or that may be developed in the future; that the market for its drug candidates may not grow at the rates anticipated or at all or that estimates for these markets may ultimately be incorrect; that Incannex may be unable to successfully execute upon any commercial discussions; Incannex's ability to comply with the various evolving and complex laws and regulations applicable to its business and its industry; and Incannex's ability to protect its proprietary technology and intellectual property; and other factors relating to Incannex's industry, its operations and results of operations. The forward-looking statements made in this press release speak only as of the date of this press release, and Incannex assumes no obligation to update publicly any such forward-looking statements to reflect actual results or changes in expectations, except as otherwise required by law. Incannex's reports filed with the U.S. Securities and Exchange Commission (SEC) including its annual report on Form 10-K for the fiscal year ended June 30, 2024, filed with the SEC on September 30, 2024, and the other reports it files from time to time, including subsequently filed annual, quarterly and current reports, are made available on Incannex's website upon their filing with the SEC. These reports contain more information about Incannex, its business and the risks affecting its business, as well as its results of operations for the periods covered by the financial results included in this press release. For additional information on Incannex, please visit our website at www.incannex.com.
Investor & Media Contacts
CORE IR
(212) 655-0924
investors@incannex.com
media@incannex.com.au