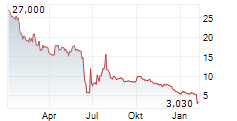
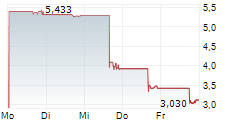
GLEN ALLEN, Va., Aug. 20, 2025 (GLOBE NEWSWIRE) -- Adial Pharmaceuticals, Inc. (NASDAQ: ADIL) ("Adial" or the "Company"), a clinical-stage biopharmaceutical company focused on developing therapies for the treatment and prevention of addiction and related disorders, today applauded recent U.S. Senate legislative support for expanding clinical trial endpoints beyond abstinence in substance use disorder treatments, including Alcohol Use Disorder (AUD). The U.S. Senate's move to encourage the FDA to develop new endpoints for AUD, such as reduced cravings, to enhance the regulatory pathway for new pharmaceutical candidates strongly reinforces Adial's clinical development strategy for AD04, its lead investigational drug, a serotonin-3 receptor antagonist, being developed for the treatment of AUD in individuals with heavy drinking and select genotypes.
The Appropriations report, which recently advanced out of Committee on a bipartisan 26-3 vote, encourages the U.S. Food and Drug Administration (FDA) and the National Institute on Drug Abuse (NIDA) to support alternative clinical endpoints such as reduced craving, reduced drug or alcohol use, and decreased disorder severity. This forward-thinking perspective validates Adial's patient-centric approach to AUD treatment and promotes a more supportive regulatory environment for AD04's continued development. By moving beyond abstinence as the singular measure of success, the new legislative language reflects real-world patient focused recovery patterns and offers an evidence-based path forward for evaluating treatments like AD04. AD04 is intended to help patients reduce alcohol intake and cravings, making it uniquely positioned to benefit from the Committee's call for more relevant, patient-focused clinical trial endpoints.
"We are encouraged by this legislative momentum, which mirrors the very foundation of our AD04 program," said Cary Claiborne, CEO of Adial Pharmaceuticals. "Our therapy is designed to reduce cravings and harmful alcohol consumption, rather than enforce an all-or-nothing abstinence model. As the U.S. Senate report makes clear, there is tremendous unmet medical need for new AUD therapies. This legislative directive signals meaningful change and momentum-one that brings the science, patient needs, and regulatory vision into closer alignment."
About Adial Pharmaceuticals, Inc.
Adial Pharmaceuticals is a clinical-stage biopharmaceutical company focused on the development of treatments for addictions and related disorders. The Company's lead investigational new drug product, AD04, is a genetically targeted, serotonin-3 receptor antagonist, being developed for the treatment of Alcohol Use Disorder (AUD). AD04 was recently investigated in a Phase 3 clinical trial ONWARD and showed promising results with reduced drinking in patients with AUD, and was well tolerated with no overt safety concerns. AD04 is also believed to have the potential to treat other addictive disorders such as Opioid Use Disorder, gambling, and obesity. Additional information is available at www.adial.com.
Forward-Looking Statements
This communication contains certain "forward-looking statements" within the meaning of the U.S. federal securities laws. Such statements are based upon various facts and derived utilizing numerous important assumptions and are subject to known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Statements preceded by, followed by or that otherwise include the words "believes," "expects," "anticipates," "intends," "projects," "estimates," "plans" and similar expressions or future or conditional verbs such as "will," "should," "would," "may" and "could" are generally forward-looking in nature and not historical facts, although not all forward-looking statements include the foregoing. The forward-looking statements include statements regarding the Committee's forward-thinking perspective validating Adial's patient-centric approach to AUD treatment and promoting a more supportive regulatory environment for AD04's continued development, developing AD04 for the treatment of AUD in individuals with heavy drinking and select genotypes, AD04 helping patients reduce alcohol intake and cravings and being uniquely positioned to benefit from the Committee's call for more relevant, patient-focused clinical trial endpoints, the legislative directive signaling meaningful change and momentum and the potential of AD04 to treat other addictive disorders such as opioid use disorder, gambling, and obesity. Any forward-looking statements included herein reflect our current views, and they involve certain risks and uncertainties, including, among others, our ability to obtain regulatory approvals for commercialization of product candidates or to comply with ongoing regulatory requirements, our ability to develop strategic partnership opportunities and maintain collaborations, our ability to obtain or maintain the capital or grants necessary to fund our research and development activities, our ability to complete clinical trials on time and achieve desired results and benefits as expected, regulatory limitations relating to our ability to promote or commercialize our product candidates for specific indications, acceptance of our product candidates in the marketplace and the successful development, marketing or sale of our products, our ability to maintain our license agreements, the continued maintenance and growth of our patent estate and our ability to retain our key employees or maintain our Nasdaq listing. These risks should not be construed as exhaustive and should be read together with the other cautionary statement included in our Annual Report on Form 10-K for the year ended December 31, 2024, subsequent Quarterly Reports on Form 10-Q and current reports on Form 8-K filed with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was initially made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise, unless required by law.
Contact:
Crescendo Communications, LLC
David Waldman / Alexandra Schilt
Tel: 212-671-1020
Email: adil@crescendo-ir.com