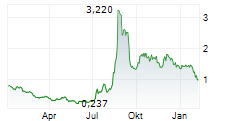
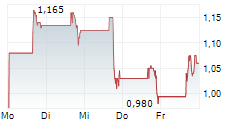
PURCHASE, N.Y., Aug. 26, 2025 (GLOBE NEWSWIRE) -- Cognition Therapeutics, Inc., (the "Company" or "Cognition") (NASDAQ: CGTX), a clinical-stage company developing drugs that treat neurodegenerative disorders, was notified by The Nasdaq Listing Qualifications Staff ("Nasdaq") that the Company has regained compliance with the exchange's continued listing standard for minimum share price under Rule 5550(a)(2) (the "Bid Price Rule").
To regain compliance with the Bid Price Rule, the Company's shares of common stock were required to maintain a minimum closing bid price of $1.00 or more for at least 10 consecutive business days, which was achieved on August 25, 2025. Accordingly, Nasdaq informed the Company that it considers the bid price deficiency matter now closed.
About Cognition Therapeutics, Inc.
Cognition Therapeutics, Inc., is a clinical-stage biopharmaceutical company discovering and developing innovative, small molecule therapeutics targeting age-related degenerative disorders of the central nervous system. We recently completed Phase 2 studies of our lead candidate, zervimesine (CT1812) in dementia with Lewy bodies (DLB), mild-to-moderate Alzheimer's disease and geographic atrophy secondary to dry AMD. The Phase 2 START study (NCT05531656) in early Alzheimer's disease is ongoing with $81 million in grant support from the National Institute of Aging (NIA) at the National Institutes of Health. We believe zervimesine can regulate pathways that are impaired in these diseases though its interaction with the sigma-2 receptor, a mechanism that is functionally distinct from other approaches for the treatment of degenerative diseases. More about Cognition Therapeutics and our pipeline can be found at https://cogrx.com.
| Contact Information: Cognition Therapeutics, Inc. info@cogrx.com | Mike Moyer (investors) LifeSci Advisors mmoyer@lifesciadvisors.com |
This press release was published by a CLEAR® Verified individual.