Real-World EMEA Physician Preference Data1 Demonstrates the SOFIA Flow 88 Aspiration Catheter's Trackability, Navigation, and Overall Performance in Stroke Procedures
MARSEILLE, France, Sept. 3, 2025 /PRNewswire/ -- Terumo Neuro, a global leader in neurovascular innovation and a wholly owned subsidiary of Terumo Corporation, today unveiled new real-world EMEA physician preference data for the SOFIA Flow 88 Aspiration Catheter at the 2025 European Society of Minimally Invasive Neurological Therapy (ESMINT) 17th Annual Congress. The data-presented publicly for the first time -demonstrates strong performance compared to other aspiration catheters1 in key performance metrics such as trackability, navigation and overall physician-rated experience.
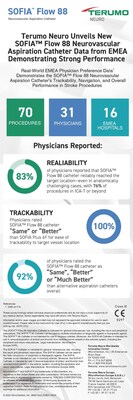
Collected from 70 procedures across 16 EMEA Hospitals and 31 physicians, the preference test revealed:
- 83% of physicians reported that SOFIA Flow 88 catheter reliably reached the target location-even in anatomically challenging cases, with 76% of procedures in ICA-T or beyond
- 100% of physicians rated SOFIA Flow 88 catheter "Same" or "Better" than SOFIA Plus 6F for ease of trackability to target vessel location
- 92% of physicians rated the SOFIA Flow 88 catheter as "Same", "Better" or "Much Better" than alternative aspiration catheters overall
"The SOFIA Flow 88 Neurovascular Aspiration Catheter represents a meaningful advancement in mechanical thrombectomy therapy across EMEA," said Carsten Schroeder, President and CEO of Terumo Neuro. "Its super bore design maximizes aspiration and minimizes flow disruption while delivering exceptional support. It enables physicians to navigate even the most tortuous anatomies in an atraumatic fashion. We're proud that physician preference data so clearly underlines SOFIA Flow 88 catheter's strong performance. It reinforces the SOFIA catheter family's reputation as 'best in class' for trackability, navigation and overall performance."
The SOFIA Flow 88 Neurovascular Aspiration Catheter was commercially launched in the EMEA on June 2, 2025, as the latest addition to Terumo Neuro's global stroke portfolio. Engineered for reliable trackability, proximal stability, and vessel-friendly flexibility, the SOFIA Flow 88 catheter is designed to optimize physician control and integration into a variety of aspiration and combination strategies.
The physician preference data unveiled at ESMINT builds on results from the U.S.-based SOFAST data and the European SESAME Study, which demonstrated the safety and efficacy of the SOFIA catheter as a frontline approach in mechanical thrombectomy.
The SOFIA Flow 88 catheter is compatible with the SOFIA Plus 6F Aspiration Catheter and is part of Terumo Neuro's fully integrated stroke solution, including:
- ERIC Retrieval Device - For thrombus control and procedural versatility
- BOBBY Balloon Guide Catheter - Offering reliable2 flow arrest with next-gen balloon technology
- WEDGE and HEADWAY Microcatheters - Designed for navigation support and access efficiency
- TRAXCESS Guidewires - Soft-tip wires built for challenging anatomies
About Terumo Neuro
We're in business to create and deliver innovations that redefine what's possible in neurovascular treatment, meaningfully advancing both physician practice and patient outcomes. Founded in 1997 as MicroVention and acquired by Terumo Corporation in 2006, Terumo Neuro offers more than thirty products for the treatment of cerebral aneurysms, ischemic stroke, carotid artery disease, and neurovascular malformations. Headquartered in California, Terumo Neuro products are sold in more than seventy countries through a direct sales organization as well as strategic distribution partnerships. Manufacturing facilities are in Aliso Viejo, California, and San José, Costa Rica. For more information, please visit www.terumoneuro.com.
About Terumo Corporation
Terumo (TSE:4543) is a global leader in medical technology and has been committed to "Contributing to Society through Healthcare" for more than 100 years. Based in Tokyo and operating globally, Terumo employs over 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company began as a Japanese thermometer manufacturer and now offers an extensive portfolio-from vascular intervention and cardio-surgical solutions to blood transfusion, cell therapy technology, and essential clinical products.
Reference
- Data on file
- Boeckh-Behrens, Tobias & Moehlenbruch, Markus. (2024). P116 The new generation BOBBY balloon guide catheter for mechanical thrombectomies: results of the international prospective STRAIT study. Journal of NeuroInterventional Surgery. 16. A97.1-A97. 10.1136/jnis-2024-ESMINT.152.
Media Contact:
Christine McCullough
Global Corporate Communications
Terumo Neuro
+1 714 206 9800
christine.mccullough@terumoneuro.com

Info - https://mma.prnewswire.com/media/2762040/Terumo_Neuro_SOFIA_Infographic.jpg
Logo - https://mma.prnewswire.com/media/2613109/Terumo_Neuro___Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/terumo-neuro-unveils-new-sofia-flow-88-neurovascular-aspiration-catheter-data-from-emea-demonstrating-strong-performance-302543901.html
View original content:https://www.prnewswire.co.uk/news-releases/terumo-neuro-unveils-new-sofia-flow-88-neurovascular-aspiration-catheter-data-from-emea-demonstrating-strong-performance-302543901.html