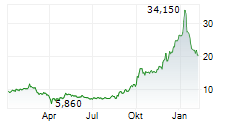
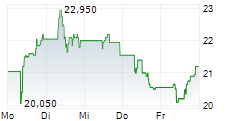
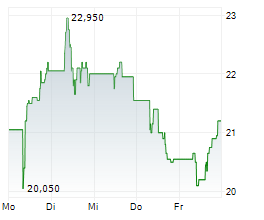
H125 saw Newron Pharmaceuticals make significant clinical headway with lead asset evenamide, culminating in the initiation of the first Phase III study, ENIGMA-TRS 1 (international study excluding the US; n=600), by period end. As of August, the first patients have been enroled (following a 42-day screening period) and the 12-week results are expected in Q426. The second study, ENIGMA-TRS 2 (international study including the US; n=400), is set to begin in October, the outcome of which will be key for US regulatory registration. Partner progress in Japan (with EA Pharma) triggered a milestone payment, which, together with the upfront consideration from Myung In Pharm (South Korea), lifted H125 licensing revenue to €7.8m (nil in H124). We expect period-end cash and equivalents of €43.2m to provide a runway into Q226 (accounting for debt repayments). Our valuation upgrades modestly to CHF407.8m or CHF20.4 per share (from CHF392.4m or CHF19.7 per share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research