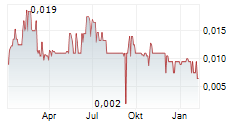
Toronto, Ontario--(Newsfile Corp. - October 9, 2025) - Psyched Wellness Ltd. (CSE: PSYC) (OTCQB: PSYCF) (FSE: 5U9) (the "Company" or "Psyched"), a life sciences company specializing in the production and distribution of health and wellness products derived from the Amanita Muscaria mushroom, is pleased to provide the following corporate update to its investors.
Product Development & Launches
Santa - Dietary Supplement Shot
Following the initial pilot run announced on April 10, 2025, the Company has made significant progress on its new dietary supplement shot, Santa.
On September 15, 2025, the Company completed an initial production run in preparation for a soft launch on October 1, 2025. A second production run is scheduled for mid-October to ensure adequate inventory for the holiday season.
Scientific Studies
On March 14, 2025, the Company initiated a Genotoxicity Study on its proprietary extract, AME-1. The study includes the AMES test, in vitro TK-MLA assay and the micronucleus assay. Progress to date has been promising, and further updates will be shared as results become available.
Additionally, we engaged a third-party laboratory to conduct a six-month accelerated stability study on AME-1. The goal was to evaluate the potential extension of product shelf life beyond the current 12-month period. Results confirm that AME-1 maintains muscimol content up to 18 months and show no microbial growth under accelerated conditions. Accordingly, Calm will now carry an 18-month shelf life.
Patents
Psyched Wellness is pleased to announce that the company submitted a "scaled-up extraction process" application in the USPTO on August 18, 2025, as U.S. Application No. 19/157,676.
The application is now pending in the U.S. and Canada.
Regulatory Update
In July 2025, the Company voluntarily submitted a New Dietary Ingredient Notification (NDIN) to the FDA for AME-1, its proprietary extract of Amanita muscaria. Though AME-1 is only derived from Amanita muscaria, the filing included over 100 years of documented food use of Amanita muscaria in a form that has not been altered along with voluntary toxicology and safety studies of AME-1 conducted by the Company. While the FDA responded in September 2025 that the specific data included in the filing by the Company did not demonstrate that AME-1 "will reasonably be expected to be safe" the Company notes that under the Federal Food, Drug, and Cosmetic Act ("FFDCA"), the purpose of an NDIN requirement is only to notify the FDA, and the submission does not require FDA concurrence or a favorable review in order for the Company to sell its products. Moreover, under the FFDCA the burden rests on FDA to prove a dietary ingredient is unsafe. The Company remains confident in AME-1's safety based on Amanita muscaria's historical use and the Company's safety studies, continues to view itself as fully compliant with FFDCA, and emphasizes its commitment to transparency and regulatory compliance as it brings additional AME-1 products to market.
Psyched Wellness will be growing their route-to-market partnerships, throughout the nation. If you are interested in distributing/listing Calm, please reach out to sales@psyched-wellness.com.
For further information, please contact:
Jeffrey Stevens
Chief Executive Officer
Psyched Wellness Ltd.
Tel: 647-400-8494
Email: jstevens@psyched-wellness.com
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
About Psyched Wellness Ltd.:
Psyched Wellness Ltd. is a Canadian-based health supplements company dedicated to researching and producing consumer packaged goods products derived from our proprietary extract of the Amanita Muscaria mushroom, AME-1.
Cautionary Statement Regarding Forward-Looking Information
This press release contains "forward-looking information" within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words "could", "proposed", "intend", "expect", "believe", "will", "projected", "estimated" and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on the Company's current belief or assumptions as to the outcome and timing of such future events. The forward-looking information and forward- looking statements contained herein include, but are not limited to, statements regarding: the ability of the Company to achieve its scheduled production runs; Company's expectations regarding regulatory compliance and market introduction of AME-1; the safety of Amanita Muscaria consumption and the safety and purity of any extracts thereof; and the uses and potential benefits of Amanita Muscaria.
Forward-looking information in this news release are based on certain assumptions and expected future events, namely: the Company's ability to continue as a going concern; the Company's ability to continue to develop its mushroom-derived products and associated consumer packaged goods; continued approval of the Company's activities by the relevant governmental and/or regulatory authorities; continued support of safety and risk assessments to support the Company's evaluation of its products; and the continued growth of the Company.
These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements, including but not limited to: the potential inability of the Company to continue as a going concern; risks associated with potential governmental and/or regulatory action with respect to the Company's operations; competition within the market; risks with respect to the safety of Amanita Muscaria consumption and the safety and purity of any extracts thereof; and the risk that there is no potential benefit of Amanita Muscaria consumption.
Readers are cautioned that the foregoing list is not exhaustive. Readers are further cautioned not to place undue reliance on forward-looking statements, as there can be no assurance that the plans, intentions or expectations upon which they are placed will occur. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated.
Forward-looking statements contained in this news release are expressly qualified by this cautionary statement and reflect the Company's expectations as of the date hereof and are subject to change thereafter. The Company undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, estimates or opinions, future events or results or otherwise or to explain any material difference between subsequent actual events and such forward-looking information, except as required by applicable law.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/269727
SOURCE: Psyched Wellness Ltd.