PRAGUE, Oct. 23, 2025 /PRNewswire/-- European innovation leader H2 Global Group today announced that its subsidiary H2 Medical Technologies has received official approval from The State Institute for Drug Control (SÚKL) to initiate a clinical study focused on molecular hydrogen inhalation therapy for patients with mild cognitive impairment (MCI), an early stage of Alzheimer's disease.
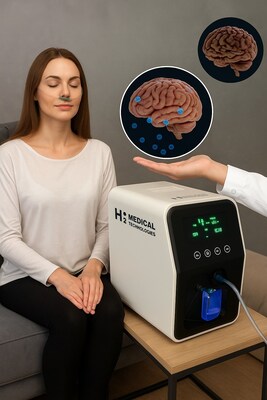
This milestone marks a historic achievement for Czech and European biotechnology, as it represents Europe's first fully approved clinical trial investigating non-invasive hydrogen inhalation as a neuroprotective therapy.
From Japanese discovery to Czech clinical practice
The project is based on pioneering research by Professor Shigeo Ohta, co-founder of H2 Global Group and widely regarded as the founding father of therapeutic hydrogen medicine. Professor Ohta personally transferred his European patent (EP 3701956 B1), titled "Prophylactic or Therapeutic Agent for Dementia," to the Group.
"Launching a clinical study that directly builds on Professor Ohta's patent is both an honor and a responsibility," said PharmDr. Milan Krajícek, co-founder of H2 Global Group. "This milestone proves that Czech innovation and research have truly a global relevance."
New hope for Alzheimer's prevention and treatment
"While several antibody-based drugs have been approved to slow Alzheimer's progression, there is still no widely available, safe, and effective non-invasive treatment," said David Maršálek, founder and CEO of H2 Global Group. "Our goal is to offer patients and physicians a gentle, accessible, and scientifically validated solution."
The study will begin in January 2026 in cooperation with Professor David Školoudík, M.D., Ph.D., one of the Czech Republic's leading neurologists. The six-month project aims to achieve the world's first regulatory registration of a medical device utilizing molecular hydrogen.
About H2 Global Group
H2 Global Group is a European innovation group connecting research, development, manufacturing, and practical application of molecular hydrogen technologies across healthcare, wellness, sports, cosmetics, and veterinary sectors.
Under the leadership of David Maršálek, the group has built a vertically integrated ecosystem, from R&D and product innovation to manufacturing and global distribution. With an estimated company valuation exceeding USD 77 million, expanding patent portfolio, and strategic partnerships worldwide, H2 Global Group ranks among Europe's most progressive biotechnology innovators. For more information, visit www.H2Global.group
Photo - https://mma.prnewswire.com/media/2803609/H2_Global_Group_protype_inhalation_H2.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/czech-breakthrough-in-medicine-h-global-group-receives-regulatory-approval-for-hydrogen-clinical-study--new-era-in-alzheimers-therapy-begins-302592725.html
View original content:https://www.prnewswire.co.uk/news-releases/czech-breakthrough-in-medicine-h-global-group-receives-regulatory-approval-for-hydrogen-clinical-study--new-era-in-alzheimers-therapy-begins-302592725.html

