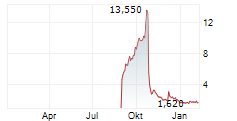
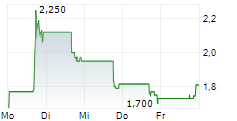
TUCSON, Ariz., Oct. 24, 2025 (GLOBE NEWSWIRE) -- Picard Medical, Inc. (NYSE American: PMI) ("Picard" or the "Company"), parent company of SynCardia Systems LLC, maker of the world's first U.S. and Canadian commercially-approved total artificial heart, today announced that it is not aware of any undisclosed material change in the Company's operations or financial condition, that would account for the recent volatility in its stock price.
The Company remains focused on executing its strategic and operational priorities and will continue to comply with all disclosure obligations under applicable securities laws.
For additional information about Picard Medical, please visit www.picardmedical.com or review the Company's filings with the U.S. Securities and Exchange Commission at www.sec.gov.
About Picard Medical and SynCardia
Picard Medical, Inc. is the parent company of SynCardia Systems, LLC ("SynCardia"), the Tucson, Arizona-based leader with the only commercially available total artificial heart technology for patients with end-stage heart failure. SynCardia develops, manufactures, and commercializes the SynCardia Total Artificial Heart ("STAH"), an implantable system that assumes the full functions of a failing or failed human heart. It is the first artificial heart approved by both the FDA and Health Canada, and it remains the only commercially available artificial heart in the United States and Canada. With more than 2,100 implants performed at hospitals across 27 countries, the SynCardia Total Artificial Heart is the most widely used and extensively studied artificial heart in the world. For more information, please visit https://www.syncardia.com
Forward-Looking Statements
This release may contain forward-looking statements within the meaning of federal securities laws. Such statements are based on current expectations and are subject to risks and uncertainties that may cause actual results to differ materially. The Company undertakes no obligation to update or revise any forward-looking statements.
Contact:
Investors
Eric Ribner
Managing Director
LifeSci Advisors LLC
eric@lifesciadvisors.com
Picard Medical, Inc./SynCardia Systems, LLC
IR@picardmedical.com
General/Media
Brittany Lanza
blanza@syncardia.com