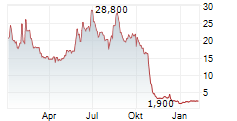
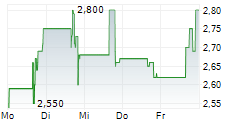
The collaboration will progress under the combined company, NewCelX, upon completion of the anticipated merger
ZURICH and NESS ZIONA, Israel, Oct. 27, 2025 /PRNewswire/ -- NLS Pharmaceutics Ltd. (Nasdaq: NLSP) ("NLS"), a Swiss clinical-stage biopharmaceutical company focused on central nervous system and neurodegenerative disorders and Kadimastem Ltd. (TASE: KDST) ("Kadimastem"), a clinical-stage biotechnology company developing breakthrough cell therapy solutions for severe diseases such as ALS and diabetes, today announced the signing of a Memorandum of Understanding ("MOU") between Kadimastem and TargetGene Biotechnologies Ltd. ("TargetGene"), a pioneer in precision gene-editing technologies.
This collaboration brings together Kadimastem's proprietary cell therapy platforms - including AstroRx® for ALS and IsletRx for diabetes - with TargetGene's next-generation, DNA-guided gene-editing platform, enabling highly specific, multiplexed genome modifications with minimal off-target activity. The collaboration is expected to continue under the combined company, NewCelX, upon completion of the anticipated merger between NLS and Kadimastem.kdst
Pursuant to the MOU, the companies will jointly develop and optimize gene-edited cell therapy products designed to enhance therapeutic performance, stability, and safety. The collaboration aims to accelerate the creation of curative, scalable, and commercially viable cell-based treatments for some of the world's most challenging diseases.
The MOU establishes a framework for joint R&D activities, technology exchange, and future licensing opportunities, forming the basis for a long-term strategic collaboration between the companies. This collaboration represents a key step in Kadimastem's strategy to expand its pipeline through next-generation gene-edited cell therapies and reinforces its commitment to delivering transformative, disease-modifying solutions to patients worldwide.
Yoel Shiboleth, Chief Executive Officer of TargetGene, said:
"Our collaboration with Kadimastem represents an exciting step forward in the evolution of cell-based medicine. By combining TargetGene's precision gene-editing technology with Kadimastem's proven stem cell platforms, we believe that we can push the boundaries of what's possible in regenerative medicine - creating safer, more effective therapies designed for long-term patient benefit."
Prof. Michel Revel, Chief Scientist and Founder of Kadimastem, said:
"The integration of precise gene editing with our stem-cell-derived therapeutic cells potentially opens new horizons in regenerative medicine. This collaboration enables us to tailor cells with enhanced biological properties, improving their survival, function, and therapeutic potential in patients with ALS and other devastating diseases."
Ronen Twito, Executive Chairman and Chief Executive Officer of Kadimastem, said:
"This collaboration with TargetGene marks an exciting leap forward in Kadimastem's mission to develop curative, cell-based therapies. By combining our advanced stem cell platforms with TargetGene's innovative gene-editing tools, we can design smarter, more resilient cells that deliver improved efficacy and long-term safety for patients."
About NLS Pharmaceutics
NLS Pharmaceutics Ltd. (Nasdaq: NLSP) is a Swiss-based biopharmaceutical company focused on the development of innovative therapies for central nervous system disorders and related indications. For more information, visit www.nlspharma.com.
About Kadimastem
Kadimastem is a clinical stage cell therapy company, developing "off-the-shelf", allogeneic, proprietary cell products based on its technology platform for the expansion and differentiation of Human Embryonic Stem Cells (hESCs) into functional cells. AstroRx®, the company's lead product, is an astrocyte cell therapy in clinical development for the treatment for ALS and in pre-clinical studies for other neurodegenerative indications.
IsletRx is the company's treatment for diabetes. IsletRx is comprised of functional pancreatic islet cells producing and releasing insulin and glucagon, intended to treat and potentially cure patients with insulin-dependent diabetes. Kadimastem was founded by Professor Michel Revel, Chief Scientific Officer of Kadimastem and Professor Emeritus of Molecular Genetics at the Weizmann Institute of Science. Professor Revel received the Israel Prize for the invention and development of Rebif®, a multiple sclerosis blockbuster drug sold worldwide. Kadimastem is traded on the Tel Aviv Stock Exchange (TASE: KDST).
About TargetGene
TargetGene Biotechnologies Ltd. is a privately held Israeli biotechnology company and a recognized pioneer in innovative gene-editing technologies. TargetGene's proprietary, best-in-class TGEE platform and genetic-circuit "rewiring" system are protected by an extensive and robust patent portfolio. At the heart of TargetGene's rewiring technologies lies its novel and proprietary super-precise TGEE programmable molecular "DNA scissors," which utilize two guide RNAs and an obligatory dimeric nuclease to achieve exceptional specificity. This approach enables safe and effective modification of a patient's own DNA, allowing precise gene deletion or gene replacement. TargetGene's versatile system supports a wide range of applications - from therapeutic genome engineering to advanced cell line development - driving innovation in the correction of genetic abnormalities, immune disorders, and cancer. The company's technology aims to enable curative genetic solutions rather than chronic treatments, offering a much safer, more efficient, and durable alternative to existing gene-editing systems such as CRISPR, which may carry risks of off-target mutations. TargetGene's platform has been recognized for its potential to redefine the future of genetic medicine by combining high specificity, modularity, and scalability suitable for clinical use.
Forward-Looking Statements
This press release contains expressed or implied forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other applicable securities laws. For example, NLS and Kadimastem are using forward-looking statements when they discuss the collaboration between Kadimastem and TargetGene, the expectation that this collaboration will continue under the combined company NewCelx, the anticipated benefits of combining Kadimastem's cell therapy platforms with TargetGene's gene-editing technology, the potential for future licensing opportunities, and the parties' intention to enter into a definitive agreement and advance toward commercialization. These forward-looking statements and their implications are based on the current expectations of the management of NLS and Kadimastem and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: risks related to the companies' ability to complete the merger on the proposed terms and schedule, including risks and uncertainties related to the satisfaction of the closing conditions related to the merger agreement; unexpected costs, charges or expenses resulting from the transaction and potential adverse reactions or changes to business relationships resulting from the completion of the proposed merger; changes in technology and market requirements; either or both companies may encounter delays or obstacles in launching and/or successfully completing their clinical trials; the companies' products may not be approved by regulatory agencies; their technologies may not be validated as they progress and their methods may not be accepted by the scientific community; either or both of the companies may be unable to retain or attract key employees whose knowledge is essential to the development of their products; unforeseen scientific difficulties may develop with the products being advanced by the companies; their products may wind up being more expensive than anticipated; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; the companies' patents may not be sufficient; their products may harm recipients; changes in legislation may adversely impact either or both of the companies; inability to timely develop and introduce new technologies, products and applications; and loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of candidate products to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, NLS does not undertake any obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting NLS is contained under the heading "Risk Factors" in NLS's annual report on Form 20-F for the year ended December 31, 2024, filed with the Securities and Exchange Commission ("SEC"), which is available on the SEC's website, www.sec.gov, and in subsequent filings made by NLS with the SEC, including under the heading "Risk Factors" in NLS's proxy statement/prospectus, filed with the SEC on September 10, 2025.
Investor & Media Contacts
NLS Contacts:
[email protected]
www.nlspharma.com
Kadimastem Contacts:
Sarah Bazak, Investors relations
[email protected]
www.kadimastem.com
Photo: https://mma.prnewswire.com/media/2805766/Kadimastem_TargetGene.jpg
Logo: https://mma.prnewswire.com/media/2637716/NLS_Pharmaceutics_Logo.jpg
SOURCE NLS Pharmaceutics Ltd.; Kadimastem Ltd.