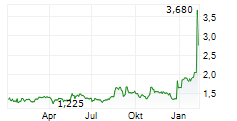
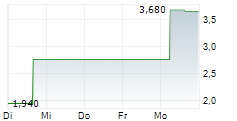
DJ Affluent Medical: Promising results for the Epygon biomimetic mitral valve presented at the TCT cardiology congress in the United States.
Affluent Medical
Affluent Medical: Promising results for the Epygon biomimetic mitral valve presented at the TCT cardiology congress in
the United States.
30-Oct-2025 / 17:45 CET/CEST
Dissemination of a French Regulatory News, transmitted by EQS Group.
The issuer is solely responsible for the content of this announcement.
=----------------------------------------------------------------------------------------------------------------------
Promising results for the Epygon biomimetic mitral valve presented at the TCT cardiology congress in the United States
-- Improvement in cardiac function after implantation of the Epygon mitral valve - preliminary clinical results on 2
patients.
-- Study of cardiac flowdynamics conducted by Dr. Mohammad Sarraf at the Mayo Clinic, MN, USA.
-- Presentation of the first clinical case at the TCT cardiology congress on October 26, 2025, in San Francisco.
Aix-en-Provence, 30 October 2025 - 5:45 p.m. CEST - Affluent Medical (ISIN: FR0013333077 - Ticker: AFME - "Affluent"),
a French clinical-stage medtech company specializing in the international development and industrialization of
innovative implantable medical devices, announces promising clinical results from two high-risk patients who underwent
implantation of the Epygon mitral valve for the treatment of severe mitral regurgitation.
The first clinical evaluation of cardiac vortex flow after implantation of the Epygon mitral valve was presented during
the Late-Breaking Clinical Science session at the TCT congress in San Francisco, USA, on October 26th 2025 by Dr.
Sarraf, interventional cardiologist at the Mayo Clinic.
These results highlight the ability of the Epygon mitral valve to preserve the natural flow dynamics of the left
ventricle, thereby improving cardiac efficiency after replacement of the native mitral valve.
Unlike most current mitral prostheses, derived from aortic valve anatomy with three leaflets that create turbulent flow
in the left ventricle, the Epygon valve was specifically designed to preserve natural blood flow. Its unique design
aims to promote the formation of a physiological vortex, essential for efficient blood flow and for reducing the
workload of the left ventricle in patients suffering from heart failure.
The study, conducted in two patients with severe mitral regurgitation, compares cardiac parameters before and three
months after implantation of the Epygon prosthesis by pressure-volume loop and ventricular bioenergetics by using
vortex analys. The results have highlighted the real added value of the Epygon valve with promising results:
-- Mitral regurgitation completely eliminated with no paravalvular leak
-- Left ventricular workload reduced by more than 50% between baseline and 3-month follow-up
-- Physiological vortex formation time (VFT), as one of the most important indices in the cardiac vortex analysis,
reduced by 53%
-- Intraventricular energy loss (EL) reduced by more than 50%.
These preliminary data demonstrate that the ability of the Epygon device to preserve left ventricular function while
improving hemodynamic performance and reducing thrombotic risk.
Dr. Sarraf, interventional cardiologist at the Mayo Clinic: "By reproducing the near-physiologic Left ventricle inflow
and restoring natural flow dynamics of the mitral valve, the Epygon device can enhance overall cardiac performance and
contribute to improve patient outcomes. This unique design combines the strong clinical performance of open-heart
surgical repair with a minimally invasive replacement approach - the best of both worlds."
Epygon mitral valve
Epygon is the only biomimetic mitral heart valve, replicating the anatomy of the native mitral valve and physiological
blood flows, implantable via a transcatheter approach. This approach avoids an invasive open-heart procedure and its
associated complications for the treatment of mitral valve insufficiency. This innovative design aims to reproduce the
natural anatomy and physiology of the native mitral valve, enabling patients to regain good cardiac function more
rapidly.
About the Minerva clinical study
The "First in Human" Minerva study is a prospective, multicenter, non-randomized, single-arm clinical trial evaluating
the minimally invasive Epygon medical device for the treatment of mitral valve regurgitation. It is currently being
conducted in seven clinical investigation centers in Italy, Spain, Hungary, Serbia, and Germany. The study will
evaluate several dozen patients to implant the Epygon valve in 10 adult patients with severe mitral regurgitation, NYHA
functional class III to IV, and a left ventricular ejection fraction (LVEF) = 30%. These patients, assessed and
selected by a multidisciplinary cardiology team, are all at high risk for open-heart mitral valve surgery and are
therefore eligible for a transcatheter repair. The objectives of the study are to assess the safety and efficacy of
Epygon valve implantation at 30 days.
About Affluent Medical
Affluent Medical is a French medtech company founded by Truffle Capital, aiming to become a global leader in the
treatment of structural heart diseases, one of the leading causes of mortality worldwide, and urinary incontinence,
which currently affects one in four adults.
Affluent Medical develops next-generation, highly innovative, minimally invasive, adjustable, and biomimetic implants
to restore essential physiological functions. The company's product candidates are all undergoing clinical studies in
humans.
For the treatment of mitral insufficiency, Affluent Medical pursues several development axes: an adjustable
beating-heart mitral ring (Kalios), a biomimetic mitral valve (Epygon)-both subject to agreements signed with Edwards
Lifesciences in July 2024 - and the company is also exploring artificial intelligence and robotics solutions for
cardiac valve implantation.
It should be noted that in June 2025, Affluent Medical raised EUR5.4 million through a convertible bond issue, fully
subscribed by its existing shareholders, enabling the company to extend its cash flow horizon until the end of the 2025
financial year. For more information, visit www.affluentmedical.com
Contacts:
AFFLUENT MEDICAL SEITOSEI.ACTIFIN
Financial communication / Press relations
Sébastien Ladet Ghislaine GASPARETTO / Jennifer JULLIA
CEO +33 (0)6 21 10 49 24 / +33 (0)1 56 88 11 19
investor@affluentmedical.com ghislaine.gasparetto@seitosei-actifin.com / jennifer.jullia@seitosei-actifin.com
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements, forecasts and estimates, including those relating to the business of Affluent Medical (the "Company"). Words such as "anticipate," "expect," "potential" and variations of such words and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements concerning the potential therapeutic benefit of the products developed by the Company as well as its cash runway. Although the Company's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks, contingencies and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. A description of these risks, contingencies and uncertainties can be found in the documents filed by the Company with the French Autorité des Marchés Financiers pursuant to its legal obligations including its universal registration document (Document d'Enregistrement Universel) filed on April 30, 2025 under number D.25-0356. These risks, contingencies and uncertainties include, among other things, the uncertainties inherent in research and development, future clinical data and analysis, decisions by regulatory authorities, such as the FDA or the EMA. Furthermore, these forward-looking statements, forecasts and estimates are made only as of the date of this press release. Readers are cautioned not to place undue reliance on these forward-looking statements. The Company disclaims any obligation to update these forward-looking statements, forecasts or estimates to reflect any subsequent changes that the Company becomes aware of, except as required by law. Information about the Company's products (including products currently in development) that is included in this press release is not intended to constitute an advertisement. This press release is for information purposes only, and the information contained herein does not constitute either an offer to sell or the solicitation of an offer to purchase or subscribe for securities of the Company in any jurisdiction. Similarly, it does not give and should not be treated as giving investment advice. It has no connection with the investment objectives, financial situation or specific needs of any recipient. It should not be regarded by recipients as a substitute for exercise of their own judgment. All opinions expressed herein are subject to change without notice. The distribution of this document may be restricted by law in certain jurisdictions. Persons into whose possession this document comes are required to inform themselves about and to observe any such restrictions.
----------------------------------------------------------------------------------------------------------------------- Regulatory filing PDF file
File: 20251030_PR_EN_Affluent Medical post TCT 2025 - final
=------------------------------------------------------------------
Language: English
Company: Affluent Medical
320 avenue Archimède, Les pléiades III Bâtiment B
13100 Aix en Provence France
France
Phone: +33 4 42 95 12 20
E-mail: jerome.geoffroy@affluentmedical.com
Internet: https://www.affluentmedical.com/
ISIN: FR0013333077
Euronext Ticker: AFME
AMF Category: Inside information / Other releases
EQS News ID: 2221510
End of Announcement EQS News Service
=------------------------------------------------------------------------------------
2221510 30-Oct-2025 CET/CEST
Image link: https://eqs-cockpit.com/cgi-bin/fncls.ssp?fn=show_t_gif&application_id=2221510&application_name=news&site_id=dow_jones%7e%7e%7ebed8b539-0373-42bd-8d0e-f3efeec9bbed
(END) Dow Jones Newswires
October 30, 2025 12:45 ET (16:45 GMT)