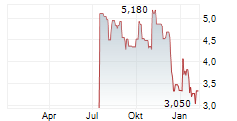
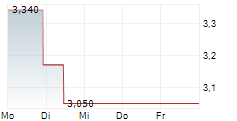
U.S. Environmental Protection Agency Initiates Public Participation Procedure
Ghent, Belgium, Oct. 31, 2025 (GLOBE NEWSWIRE) -- Press release - inside information
EPA Issues Proposed Registration Decision for Biotalys' EVOCA Biofungicide
U.S. Environmental Protection Agency Initiates Public Participation Procedure
Ghent, BELGIUM - October 31, 2025, 7.00 CET - Biotalys) is pleased to announce that the United States Environmental Protection Agency (EPA) has issued its proposed registration decision to approve the company's first biofungicide, EVOCA*. The agency will now initiate the final phase in its regulatory review, allowing stakeholders such as growers and industry associations 15 days to provide feedback before finalizing its regulatory decision.
The EPA has also posted a final rule exempting EVOCA's active ingredient residues on treated crops from tolerance requirements, indicating that no maximum residue limits will apply and highlighting the product candidate's exemplary safety profile.
EVOCA is a precision biocontrol solution that targets the fungal pathogens botrytis (grey mold) and powdery mildew in high-value fruits and vegetables without harming beneficial organisms or the environment. The product candidate offers growers a new mode of action to manage resistance to existing fungicides as was recognised by the Fungicide Resistance Action Committee (FRAC)**, a panel of renown industry experts.
"The EPA's recommendation to approve EVOCA marks a pivotal moment for Biotalys, and the advancement of the first protein-based biofungicide of its kind for approval in the United States. This achievement underscores Biotalys' pioneering leadership in agricultural innovation and provides the company with a significant first-mover advantage in transforming how growers protect their crops safely and sustainably." said Kevin Helash, CEO of Biotalys.
"Our protein-based platform has the potential to transform crop protection by delivering next generation biological solutions with new modes of action, developed to create targeted, consistent and scalable products to meet farmers' needs, as demonstrated today by the EPA's publication," he added.
"This significant milestone reflects the dedication and expertise of the entire Biotalys team," Helash concluded. "EVOCA is just the beginning for Biotalys, and we look forward to bringing our pipeline of fungicides and insecticides to the market in the coming years."
An approval of EVOCA paves the way for the regulatory submission of EVOCA NG, the next-generation version of the product candidate, and Biotalys' anticipated first commercial launch. The company expects the review process for EVOCA NG to be significantly shorter as the product contains the same active ingredient as EVOCA and features enhanced formulation and production methods.
To read the full EPA documents please visit:
https://www.regulations.gov/docket/EPA-HQ-OPP-2021-0165
https://www.federalregister.gov/public-inspection/2025-19712/pesticide-tolerance-exemptions-petitions-revocations-etc-asfbiof01-02-polypeptide
* EVOCA: Pending regulatory review. This product candidate is not currently registered for sale or use in the United States, the European Union, or elsewhere and is not being offered for sale and no assurance can be given if, when and under which conditions registration will be obtained.
** EVOCA obtained FRAC-code F10.
About Biotalys
Biotalys is an Agricultural Technology.

For further information, please contact:
Toon Musschoot, Head of IR & Communication
T: +32
Important Notice
Biotalys, its business, prospects and financial position remain exposed and subject to risks and uncertainties. A description of and reference to these risks and uncertainties can be found in the annual reporton the consolidated annual accounts published on the company's website.
This announcement contains statements which are "forward-looking statements" or could be considered as such. These forward-looking statements can be identified by the use of forward-looking terminology, including the words 'aim', 'believe', 'estimate', 'anticipate', 'expect', 'intend', "have the potential", 'may', 'will', 'plan', 'continue', 'ongoing', 'possible', 'predict', 'plans', 'target', 'seek', 'would' or 'should', and contain statements made by the company regarding the intended results of its strategy. By their nature, forward-looking statements involve risks and uncertainties and readers are warned that none of these forward-looking statements offers any guarantee of future performance. Biotalys' actual results may differ materially from those predicted by the forward-looking statements. Biotalys makes no undertaking whatsoever to publish updates or adjustments to these forward-looking statements, unless required to do so by law.
Attachments
- Biotalys Persbericht - Evoca Publiek Commentaar en torlerantie (WD) PDF (https://ml.globenewswire.com/Resource/Download/1ada8e5b-53b7-4005-9e21-0343eb6ead94)
- Biotalys Press release FInal PDF (https://ml.globenewswire.com/Resource/Download/62cedbd5-989a-440e-8bb4-f66daff61cd1)