- Fast Track Designation was granted for BB-301 following FDA review of positive interim clinical study results and proprietary Responder Analysis planned for use in pivotal study for BB-301
- BB-301 has also been granted Orphan Drug Designation from both FDA and EMA
- All six patients enrolled into Cohort 1 met the formal statistical criteria for response to BB-301, representing a 100% response rate
- Following the administration of BB-301, Cohort 1 patients experienced significant continuing reductions in dysphagic symptom burden, post-swallow residue accumulation, time required to consume fixed volumes of liquid, and improved pharyngeal closure during swallowing
- First patient in Cohort 2 successfully treated with BB-301 in fourth quarter of 2025
- Benitec plans to meet with the FDA in 2026 to confirm the BB-301 pivotal study design
- Dr. Sharon Mates, who served as Chairman, Chief Executive Officer, and Co-founder of Intra-Cellular Therapies Inc., appointed to the Benitec Biopharma Board of Directors as previously disclosed
HAYWARD, Calif., Nov. 03, 2025 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) ("Benitec" or "Company"), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary "Silence and Replace" DNA-directed RNA interference ("ddRNAi") platform, today provides positive interim clinical results for the BB-301 Phase 1b/2a Clinical Trial. Following administration of BB-301, Cohort 1 patients demonstrated significant and sustained improvements across multiple clinical measures including dysphagic symptom burden, post-swallow residue accumulation, time required to consume fixed volumes of liquid, as well as improved pharyngeal closure during swallowing. All six patients enrolled into Cohort 1 met the formal statistical criteria for response to BB-301, representing a 100% response rate. Following review of these encouraging interim data, the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to BB-301 for the treatment of OPMD with dysphagia. BB-301 was also previously granted Orphan Drug Designation from both the FDA and European Medical Association (EMA).
"Progressive dysphagia is a severe, life-threatening complication of OPMD which impacts 97% of OPMD patients, often leading to serious health risks, such as chronic choking, malnutrition, aspiration pneumonia, and death. We are excited by the profound effect that BB-301 can potentially have on this progressive disease as demonstrated by the interim clinical trial results for Cohort 1, where 100% of patients were responders" said Jerel A. Banks, M.D., Ph.D., Executive Chairman and Chief Executive Officer of Benitec Biopharma Inc. "Securing Fast Track designation for BB-301 reflects the strength of our clinical data and the urgency of the unmet need in OPMD. This recognition validates our team's scientific and strategic execution, and we look forward to continued collaboration with the FDA as we advance toward a pivotal clinical trial."
The pre-treatment data for Cohort 1 patients reflect the first six months of Natural History Study follow-up and the final pre-treatment visit (i.e., the Phase 1 Screening Visit)
The interim post-treatment data for Cohort 1 patients reflect the following:
- 12-months of post-BB-301-treatment follow-up for Patient 1 and Patient 2
- 9-months of post-BB-301-treatment follow-up for Patient 3
- 6-months of post-BB-301-treatment follow-up for Patient 4 and Patient 5; and
- 3-months of post-BB-301-treatment follow-up for Patient 6
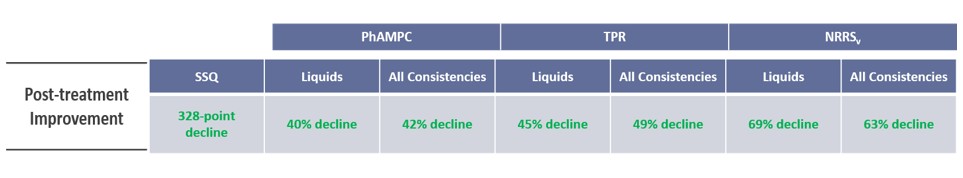
As the total dysphagic symptom burden experienced by OPMD patients has several known underlying contributors, the development of a multi-component composite endpoint to evaluate the potential treatment effects of BB-301 allows for incorporation of multiple discrete assessments that, in total, assess disease progression and treatment benefit of BB-301.
The BB-301 Responder Analysis (the multi-component composite endpoint) is comprised of a combination of patient-reported outcome results, objective assessment results, and swallowing capacity assessment results:
- Patient-Reported Outcome assessment results include: Sydney Swallow Questionnaire or "SSQ" results
- Objective Assessment Results include: Videofluoroscopic swallowing study results (Pharyngeal Area at Maximum Constriction or "PhAMPC", Post-Swallow Pharyngeal Residue as measured by Total Pharyngeal Residue or "TPR" and Normalized Residue Ratio Scale or "NRRS", Frequency of sequential swallows or "SEQ")
- Functional Swallowing Capacity Assessment Results include: Clinically administered drinking assessment results (as measured by the cold-water timed drinking test or "CWDT")
Following the administration of BB-301, Cohort 1 patients experienced clinically significant reductions, and met the formal statistical criteria for response, in the following assessments:
Summary of Cohort 1 Results
To date, the Benitec OPMD Natural History Study and the BB-301 Phase 1b/2a Clinical Trial represent the only clinical studies ever conducted which employ serial evaluation of the dysphagic symptom burden of OPMD patients and serial radiographic evaluation of the anatomical and functional elements of swallowing in OPMD patients at a frequency of approximately every 3-months. Positive interim clinical study results demonstrate the significant and durable clinical benefit achieved by patients treated with BB-301.
Company Webcast Information:
Webcast title: Interim BB-301 Phase 1b/2a Clinical Study Update
A live webcast of the interim clinical data presentation, will be held at 8:00 AM ET on Monday, November 3, 2025, and can be accessed by clicking here.
The event replay and corresponding slides will be placed on the News & Events tab on the Investor page of the Benitec website.
About BB-301
BB-301 is a novel, modified AAV9 capsid expressing a unique, single bifunctional construct promoting co-expression of both codon-optimized Poly-A Binding Protein Nuclear-1 (PABPN1) and two small inhibitory RNAs (siRNAs) against mutant PABPN1 (the causative gene for OPMD). The two siRNAs are modeled into microRNA backbones to silence expression of faulty mutant PABPN1, while allowing expression of the codon-optimized PABPN1 to replace the mutant with a functional version of the protein. We believe the silence and replace mechanism of BB-301 is uniquely positioned for the treatment of OPMD by halting mutant expression while providing a functional replacement protein. BB-301 has received Orphan Drug Designation from the EMA and Orphan Drug and Fast Track Designations from the FDA.
About Benitec Biopharma, Inc.
Benitec Biopharma Inc. ("Benitec" or the "Company") is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary "Silence and Replace" DNA-directed RNA interference platform combines RNA interference, or RNAi, with gene therapy to create medicines that simultaneously facilitate sustained silencing of disease-causing genes and concomitant delivery of wildtype replacement genes following a single administration of the therapeutic construct. The Company is developing Silence and Replace-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD). A comprehensive overview of the Company can be found on Benitec's website at www.benitec.com.
Forward Looking Statements
Except for the historical information set forth herein, the matters set forth in this press release include forward-looking statements, including statements regarding Benitec's plans to develop and commercialize its product candidates and the clinical utility and potential attributes and benefits of ddRNAi and Benitec's product candidates, and other forward-looking statements.
These forward-looking statements are based on the Company's current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the success of our plans to develop and potentially commercialize our product candidates; the timing of the completion of preclinical studies and clinical trials; the timing and sufficiency of patient enrollment and dosing in any future clinical trials; the timing of the availability of data from our clinical trials; the timing and outcome of regulatory filings and approvals; the development of novel AAV vectors; our potential future out-licenses and collaborations; the plans of licensees of our technology; the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a "one shot" cure; our intellectual property position and the duration of our patent portfolio; expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of patients in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company's ability to protect and enforce its patents and other intellectual property rights; the Company's dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company's products and the products of the Company's collaboration partners; the acceptance of the Company's products and the products of the Company's collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting, the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company's reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements.
Investor Relations Contact:
Irina Koffler
LifeSci Advisors, LLC
(917) 734-7387
ikoffler@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/82289b3c-6338-4e34-9274-b0507edf6346