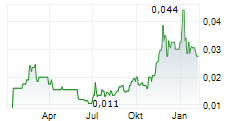
Actinogen Medical reported that the independent Data Monitoring Committee (DMC) of the company's pivotal Phase IIb/III XanaMIA study in patients with mild-to-moderate Alzheimer's disease (AD) has met for the first time, and it has recommended that the study continues without modifications. The DMC has reviewed all available safety data to date from 153 XanaMIA study participants and has determined that no study modifications are indicated. This suggests that the drug continues to be well-tolerated and that its safety profile remains very favourable, which could be a key differentiator (provided it receives eventual regulatory approval), given well-recognised safety concerns with anti-amyloid treatments in AD. Actinogen plans to report a pre-planned interim efficacy (futility) analysis in early Q1 CY26 and final top-line study data in mid-Q4 CY26.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research