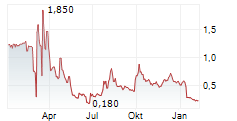
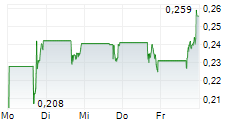
HOUSTON, Nov. 17, 2025 (GLOBE NEWSWIRE) -- Plus Therapeutics, Inc. (Nasdaq: PSTV) ("Plus" or the "Company"), a clinical-stage pharmaceutical company developing targeted radiotherapeutics with advanced platform technologies for central nervous system (CNS) cancers, today announces it has received an additional 180-day extension period from the Nasdaq Listing Qualifications Department (the "Nasdaq Staff") to regain compliance with the $1.00 minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) (the "Minimum Bid Price Requirement").
The extension notice has no immediate effect on the continued listing status of the Company's common stock on the Nasdaq Capital Market ("Nasdaq") under the symbol "PSTV." The Company has until May 11, 2026, to meet the Minimum Bid Price Requirement. If at any time during the additional 180-calendar-day extension, the closing bid price of the common stock is at least $1.00 per share for a minimum of 10 consecutive business days, the Nasdaq Staff will provide the Company with a written confirmation of compliance, and the matter will be closed.
There can be no assurance that the Company will be able to regain compliance with the Minimum Bid Price Requirement, even if it maintains compliance with the other listing requirements.
About Plus Therapeutics
Headquartered in Houston, Texas, Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing targeted radiotherapeutics for difficult-to-treat cancers of the central nervous system with the potential to enhance clinical outcomes. Combining image-guided local beta radiation and targeted drug delivery approaches, the Company is advancing a pipeline of product candidates with lead programs in leptomeningeal metastases (LM) and recurrent glioblastoma (GBM). The Company has built a supply chain through strategic partnerships that enable the development, manufacturing, and future potential commercialization of its products. For more information, visit https://www.plustherapeutics.com.
About CNSide Diagnostic, LLC
CNSide Diagnostics, LLC is a wholly owned subsidiary of Plus Therapeutics, Inc. that develops and commercializes proprietary laboratory-developed tests, such as CNSide®, designed to identify tumor cells that have metastasized to the central nervous system in patients with carcinomas and melanomas. The CNSide® CSF Assay Platform enables quantitative analysis of the cerebrospinal fluid that informs and improves the management of patients with leptomeningeal metastases.
Forward-Looking Statements
This press release contains statements that may be deemed "forward-looking statements" within the meaning of U.S. securities laws, including the Company's ability to maintain compliance with the Minimum Bid Price Requirement and other Nasdaq's continued listing rules. All statements in this press release other than statements of historical fact are forward-looking statements. These forward-looking statements may be identified by future verbs, as well as terms such as "expect," "will," "potential," "anticipating," "planning," and similar expressions or the negatives thereof. Such statements are based upon certain assumptions and assessments made by management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. The forward-looking statements included in this press release could differ materially from those expressed or implied by these forward-looking statements because of risks, uncertainties, and other factors that include, but are not limited to, the following: the price and volatility of the Company's common stock; the Company's liquidity and capital resources and its ability to raise additional cash; market conditions, product performance, litigation or potential litigation; and, competition within the cancer diagnostics and therapeutics field. This list of risks, uncertainties, and other factors is not complete. The Company discusses some of these matters more fully, as well as certain risk factors that could affect the Company's business, financial condition, results of operations, and prospects, in its reports filed with the SEC, including the Company's annual report on Form 10-K for the fiscal year ended December 31, 2024, quarterly reports on Form 10-Q, and current reports on Form 8-K. These filings are available for review through the SEC's website at www.sec.gov. Any or all forward-looking statements the Company makes may turn out to be wrong and can be affected by inaccurate assumptions the Company might make or by known or unknown risks, uncertainties, and other factors, including those identified in this press release. Accordingly, you should not place undue reliance on the forward-looking statements made in this press release, which speak only as of its date. There may be events in the future that the Company is unable to predict, or over which it has no control, and its business, financial condition, results of operations and prospects may change in the future. The Company assumes no responsibility to update or revise any forward-looking statements to reflect events, trends or circumstances after the date they are made unless the Company has an obligation under U.S. federal securities laws to do so.
Investor Contact
CORE IR
investor@plustherapeutics.com