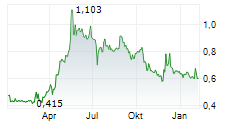
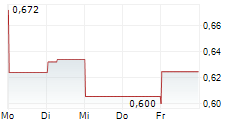
Cereno Scientific has announced that it has been granted FDA clearance for its global Phase IIb trial for CS1 in pulmonary arterial hypertension (PAH), clearing the way for trial initiation in Q226. Management also provided details on the study design, which will be a 126-patient, double-blind, placebo-controlled, dose-finding trial, assessing two dose levels of CS1 versus placebo in combination with background therapy. We note the 60-week total treatment duration (including screening and follow-up) with a re-randomisation after 36-weeks, ensuring all participants receive active treatment at some point during the trial. We believe this to be strategic, allowing the company to confirm previously observed disease-modifying signals in a larger, controlled cohort. Study endpoints include change in pulmonary vascular resistance (PVR) and six-minute walk distance (6MWD) at week 36 (well-validated regulatory benchmarks in PAH), with top-line readouts expected in Q428. We will review our estimates to reflect this news and will present our updated valuation in a forthcoming note.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research