PONTE VEDRA, FL / ACCESS Newswire / December 12, 2025 / Cadrenal Therapeutics (NASDAQ:CVKD) entered the back half of the year with a calm, almost surgical approach to building out its foundation. While most microcaps lean on noise to stay visible, Cadrenal has let its work do the lifting. Trial preparation moved forward. Manufacturing advanced. Leadership depth expanded inside the clinical ranks. None of it screamed for attention, but each decision made the Company harder to ignore. Quiet progress is still progress, and in Cadrenal's case, it set the tone for everything that followed.
Tecarfarin, one of the Company's drug candidates, sits in one of the most clinically neglected segments of anticoagulation. High-risk patients with end-stage kidney disease and complex cardiac burdens simply do not get the predictable outcomes they should from today's drugs. Tecarfarin was designed to address that gap with control, reversibility, and stability that align with real patient needs.
What makes Cadrenal's approach interesting is that none of this has been hyped. The Company has acted as a team focused on execution first. It spent months laying the groundwork, putting the structure in place so trials could progress to key milestones. Companies that prepare this thoughtfully usually do it for one reason. They expect their next phase to be bigger than the market expects.
A Pipeline That Grew While No One Was Looking
Then came the expansion. Cadrenal added the Factor XIa program, designed to provide a new pathway into acute hospital care, where safer, more controlled anticoagulation would be welcomed immediately. This was not a cosmetic acquisition. It broadened the Company's mechanistic range and created opportunities that complement tecarfarin rather than compete with it. Suddenly, Cadrenal had a presence in both chronic and acute environments.
The next move lifted the floor even higher. Cadrenal just acquired VLX-1005, a Phase 2 asset with Orphan Drug and Fast Track designations for heparin-induced thrombocytopenia (HIT). HIT is rare, dangerous, and urgently needs better options. A company of Cadrenal's size acquiring a program with that kind of regulatory positioning is uncommon. It is strategic. It puts Cadrenal in conversations that simply were not available to it a few quarters ago.
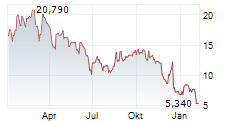
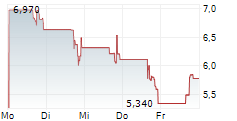
All of this portfolio building happened while the stock continued to trade as if nothing had changed. The valuation could reflect an outdated picture, not the broader platform the Company now controls. That disconnect could create opportunity. Eventually, the market must realign because pipelines spanning multiple treatment settings do not remain hidden in microcap territory once clinical momentum builds.
The Moment Before Momentum
Cadrenal is entering the stretch where quiet groundwork could turn into visible catalysts. Trial preparations are lining up. Program progression is advancing. Regulatory engagements start to matter more. Each step has the potential to reshape how investors view the Company. This is where a steady, disciplined strategy could pay off. The Company has done heavy lifting behind the scenes. Now the cycle shifts toward execution that the market can actually see.
The portfolio itself is structured to create leverage. Tecarfarin's chronic care positioning addresses the real-world needs of underserved patients for decades. The Factor XIa program moves Cadrenal into high-value hospital environments. VLX-1005 enters a space where even incremental improvement would command serious attention. Each asset strengthens the others by showing a company with a coherent vision rather than a scattershot pipeline.
The quiet phase appears to be ending. Cadrenal built a platform that speaks louder with data than with headlines. CVKD still appears small on the screen, but the underlying architecture suggests otherwise. This is not a company waiting for relevance. It is a company preparing to express itself.
About Cadrenal Therapeutics, Inc.
Cadrenal Therapeutics, Inc. is developing differentiated products that bridge critical gaps in current acute and chronic anticoagulation management for rare and high-risk patient populations. It currently has three clinical-stage assets: VLX-1005, a first-in-class Phase 2 12-LOX Inhibitor for patients with HIT, tecarfarin, an oral vitamin K antagonist (VKA) for chronic use in patients with kidney dysfunction or left ventricular assist devices (LVADs), and frunexian, a parenteral small-molecule Factor XIa antagonist for use in acute hospital settings. For more information, visit https://www.cadrenal.com/ and connect with the Company on LinkedIn.
Safe Harbor
Any statements in this press release about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute "forward-looking statements." The words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potentially," "predict," "project," "should," "target," "will," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements include statements regarding Tecarfarin being designed to address that gap in predictable outcomes for high-risk patients with end-stage kidney disease and complex cardiac burdens with control, reversibility, and stability that align with real patient needs; spending months laying the groundwork, putting the structure in place so trials could progress to key milestones; companies expecting the next phase to be bigger than the market expects; the Factor XIa program providing a new pathway into acute hospital care, the valuation of the stock creating opportunity; the market eventually realigning; clinical momentum building; Cadrenal entering the stretch where quiet groundwork could turn into visible catalysts; trial preparations lining up; program progression beginning; regulatory engagements starting to matter more; each step having the potential to reshape how investors view the Company; this being where a steady, disciplined strategy could pay off; Tecarfarin's chronic care positioning addressing the real-world needs of underserved patients for decades; the quiet phase appearing to be ending; CVKD still appearing small on the screen, but the underlying architecture suggesting otherwise; and being a company preparing to express itself.. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, the ability to continue to advance novel therapeutics to treat or prevent thrombosis in high-risk patients; including the ability to advance the clinical development of VLX-1005 for the treatment of HIT; the ability to use the acquisition of a Factor XIa portfolio to provide a new pathway to acute hospital care; the ability to successfully complete clinical trials on time and achieve desired results and benefits as expected; the ability to obtain regulatory approvals for commercialization of product candidates or to comply with ongoing regulatory requirements and the other risk factors described in the Company's Annual Report on Form 10-K for the year ended December 31, 2024, and the Company's subsequent filings with the Securities and Exchange Commission, including subsequent periodic reports on Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. Any forward-looking statements contained in this press release speak only as of the date hereof and, except as required by federal securities laws, the Company specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events, or otherwise.
Accuracy & Disclosure Statement: Hawk Point Media Group, LLC (HPM) has been retained by IR Agency, Inc. to provide press releases, editorial insights, and digital media production for Cadrenal Therapeutics. This content is sponsored. For services rendered from December 12, 2025 through December 16, 2025, HPM has been compensated ten thousand dollars (USD) via wire transfer for content creation and syndication related to Cadrenal Therapeutics. The information contained herein is based on sources believed to be accurate and reliable at the time of creation, including publicly available filings, company disclosures, and direct website content. This material is provided for informational purposes only and should not be interpreted as investment advice, a recommendation, or an offer to buy or sell any security.
At the time of publication, HPM does not own, buy, sell, or trade securities of the companies covered. However, individuals or organizations that have retained HPM may hold shares of Cadrenal Therapeutics and may sell those shares during the coverage period. Such sales could place downward pressure on the stock price and result in financial loss for investors.
Any reproduction, redistribution, or syndication of this content must include this disclosure in full. This statement is provided in accordance with Section 17(b) of the Securities Act of 1933, the Federal Trade Commission's Endorsement Guides, and other applicable laws governing sponsored communications and paid investor content.
Contact for this content: info@hawkpointmedia.com
SOURCE: Cadrenal Therapeutics
View the original press release on ACCESS Newswire:
https://www.accessnewswire.com/newsroom/en/healthcare-and-pharmaceutical/cadrenals-quiet-expansion-play-is-starting-to-get-loud-1117023