NEW YORK CITY (dpa-AFX) - Bristol Myers Squibb (BMY) reported positive topline data from the Phase 3 SCOUT-HCM trial, the first randomized, placebo-controlled study of a cardiac myosin inhibitor in adolescents aged 12 to under 18 with symptomatic obstructive hypertrophic cardiomyopathy.
The study met its primary endpoint, showing a statistically significant reduction in Valsalva left ventricular outflow tract gradient at 28 weeks compared with placebo, indicating improved obstruction. Several secondary endpoints tied to disease symptoms and functional measures were also met. Safety findings were in line with the established adult profile of Camzyos, with no new safety signals seen in the adolescent population.
The company leadership said the results support the potential for Camzyos to become the first cardiac myosin inhibitor approved for adolescents with obstructive HCM, an area where treatment options are currently limited to symptom management or invasive procedures. Investigators highlighted the importance of the trial for pediatric cardiology, noting that few late-stage, placebo-controlled studies in this field have produced positive outcomes.
Bristol Myers Squibb plans to present detailed data at a future medical meeting and discuss the findings with regulators. The SCOUT-HCM study enrolled 44 patients worldwide and includes long-term extension phases that will continue to track efficacy and safety.
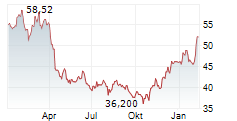
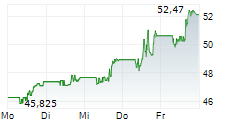
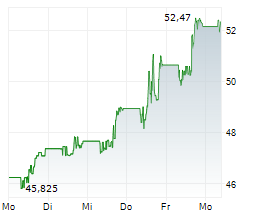
BMY currently trades at $55.71, or 0.27% lower on the NYSE.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News